Mannanase pman5a mutant with improved thermotolerance and its gene and application
A mannanase and mutant technology, applied in the field of agricultural biology, can solve the problems of low catalytic activity, poor β-mannanase tolerance and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Embodiment 1 Construction of mannanase mutant engineering bacteria
[0064] (1) Construction of expression vector and expression in Pichia pastoris
[0065] Using the GH5 family mannanase Pman5A-pPIC9 derived from Penicillium sp.WN1 as a template, mutant primers H93Y F / R, F94Y F / R, L356H F / R and For A389P F / R (as shown in Table 1), the point mutation kit was used to perform PCR amplification first, then the PCR product was demethylated by DMTase and transformed into DMT competent cells, and finally the single clone was selected for verification and positive selection The transformants were subjected to DNA sequencing, and the transformants with the correct sequence were used for large-scale preparation of recombinant plasmids.
[0066] Table 1 takes wild mannanase PMan5A as the primers used to construct mutants
[0067]
[0068] Linearize the recombinant vector with the correct sequencing of the expression vector pPIC9 with endonuclease DraI and transform it into P...
Embodiment 2
[0071] Embodiment 2 Mannanase mutants and the preparation of wild enzyme enzyme liquid
[0072] (1) Expression of mutant genes at shake flask level in Pichia pastoris
[0073] Pick the transformant with the highest enzyme activity, inoculate it in 30mL YPD medium and culture it for 48h for seed scale-up cultivation, then inoculate it into a 1L Erlenmeyer flask with 300mL BMGY medium according to the inoculation volume of 1%, and culture it on a shaker at 30°C and 220rpm After 48 hours, the culture solution was centrifuged at 3000 g for 5 minutes, the supernatant was discarded, and the pellet was resuspended in 200 mL of BMMY medium containing 0.5% methanol, and placed again at 30°C and 220 rpm to induce culture. Add 1 mL of methanol every 12 hours to keep the concentration of methanol in the bacterial solution at 0.5%, and take the supernatant for enzyme activity detection.
[0074] (2) Purification of mutant mannanase
[0075] Collect the supernatant of the mutant mannanase...
Embodiment 3
[0076] Example 3 Activity analysis and property determination of mannanase mutants and wild enzyme Pman5A
[0077] Adopt DNS method to carry out activity analysis to mannanase of the present invention, concrete method is as follows:
[0078] Under the given pH and temperature conditions, 1 mL of the reaction system includes 100 μL of appropriate diluted enzyme solution, 900 μL of substrate, reacted for 10 min, added 1.5 mL of DNS to terminate the reaction, and boiled for 5 min. After cooling, the OD value was measured at 540 nm. One enzyme activity unit (U) is defined as the amount of enzyme required to decompose carob gum to produce 1 μmol reducing sugar per minute under given conditions.
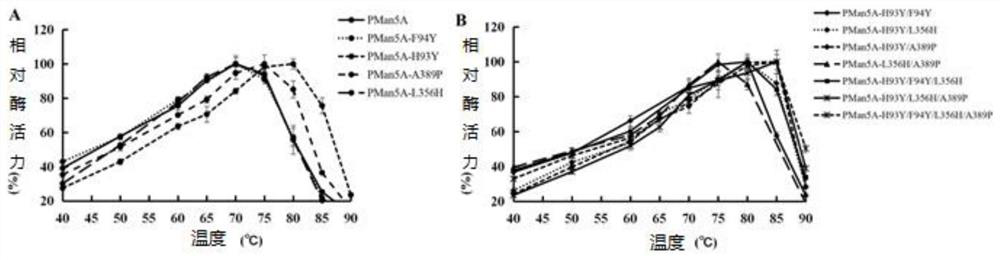
[0079] (1) Determination of optimum temperature and temperature stability of mutant and wild enzyme Pman5A
[0080] The enzyme-catalyzed reaction was carried out in a pH5.0 citric acid-disodium hydrogen phosphate buffer system and at different temperatures (40-90° C.), and the optimum te...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com