Method for synthesizing other amiprid from amiprid
A technology for the synthesis and compound of amidines, applied in the direction of organic chemistry, etc., can solve the problems of cumbersome product post-treatment, harsh reaction conditions, long reaction time, etc., and achieve the effect of simple product separation and purification, mild reaction conditions, and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
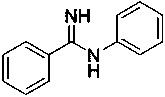
[0025] The preparation of N-phenyl benzamidine, structural formula is as follows:
[0026]
[0027] Under nitrogen protection, the raw material N-methyl-N-phenylbenzamidine (0.5 mmol) and the catalyst Lu(CH 2 Ph) 3 (10mol%), aniline (1 mL), reacted at 60 °C for 12 h, and the isolated yield of the product was 91%.
[0028] 1 H NMR (400 MHz, CDCl3): δ 7.87-7.86 (m, 2H), 7.50-7.43 (m, 3H), 7.40-7.36 (m, 2H), 7.11-7.07 (m, 1H), 7.01-7.00 ( m, 2H), 4.93 (s, 2H).
Embodiment 2
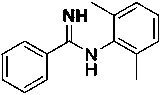
[0030] The preparation of N-(2,6-dimethylphenyl) benzamidine, the structural formula is as follows:
[0031]
[0032] Under nitrogen protection, the raw material N-methyl-N-phenylbenzamidine (0.5 mmol) and the catalyst Lu(CH 2 Ph) 3 (10mol%), 2,6-dimethylaniline (1 mL), reacted at 60 °C for 12 h, and the isolated yield of the product was 83%.
[0033] 1 H NMR (400 MHz, CDCl3): δ 7.87-7.85 (m, 2H), 7.45-7.38 (m, 3H),7.05-7.03 (m, 2H), 6.90-6.87 (m, 1H), 4.60 (s, 2H), 2.13 (s, 6H).
Embodiment 3
[0035] The preparation of N-(2,6-dimethylphenyl)-4-methylbenzamidine, the structural formula is as follows:
[0036]
[0037] Under nitrogen protection, the raw material N-methyl-N-phenylbenzamidine (0.5 mmol) and the catalyst Lu(CH 2 Ph) 3 (10mol%), 4-methylaniline (1 mL), reacted at 60 °C for 12 h, and the isolated yield of the product was 76%.
[0038] 1 H NMR (400 MHz, CDCl3): δ 7.75-7.73 (m, 2H), 7.19-7.17 (m, 2H),7.03-7.01 (m, 2H), 6.89-6.85 (m, 1H), 4.57 (s, 2H), 2.37 (s, 3H), 2.11 (s, 6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com