Hydrobromide of benzodiazepine derivative, preparation method and uses thereof
一种氢溴酸盐、氢溴酸的技术,应用在医药化学领域,能够解决临床使用不便、药物剂量不准确、长时间震荡溶解等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] The preparation of the hydrobromide III crystal form of embodiment 1 formula (I) compound
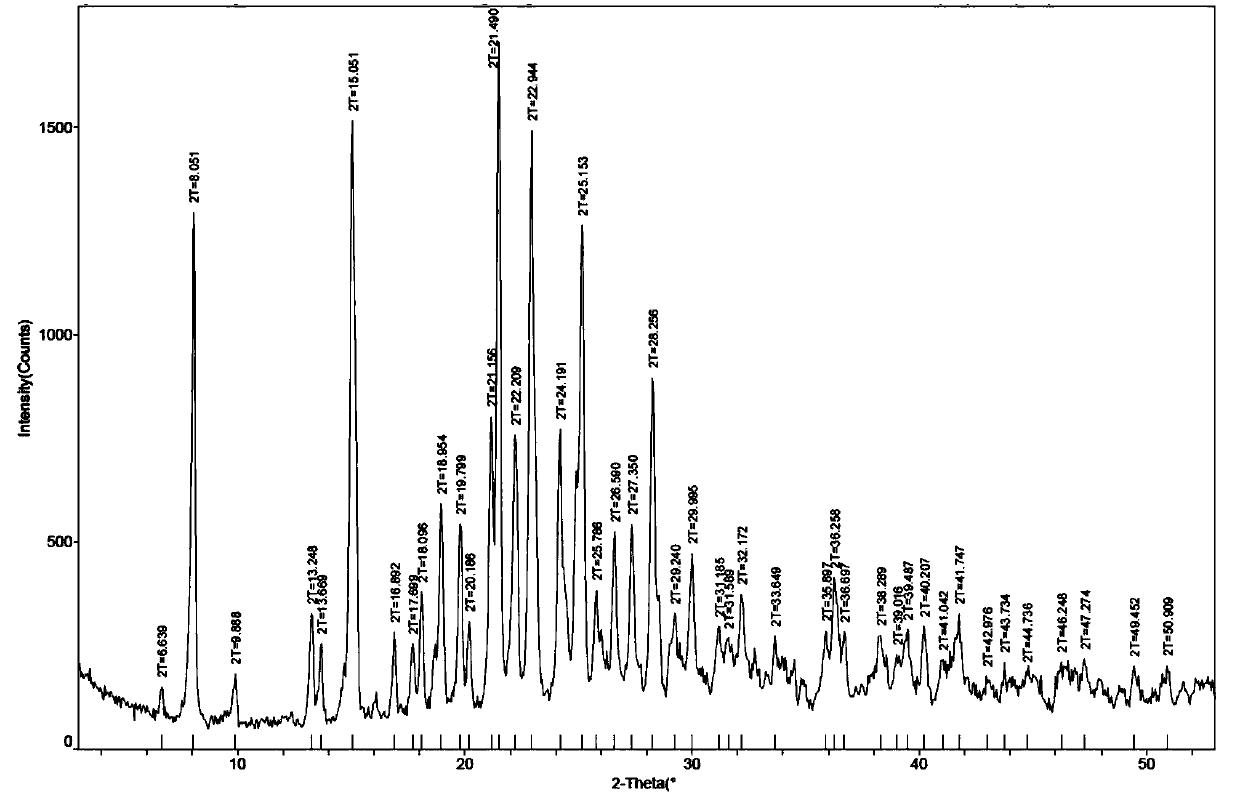
[0070] Accurately weigh 1.8g of the compound of formula (I) in a 100mL there-necked flask, add 8.2mL of isopropanol and stir to make it all dissolve, then dissolve 0.83g of 47% aqueous hydrobromic acid in 6.3mL of isopropanol, and drop Add it to the isopropanol solution of the compound of formula (I), stir and crystallize, filter, and dry under reduced pressure at 55°C to obtain the hydrobromide salt of the compound of formula (I).
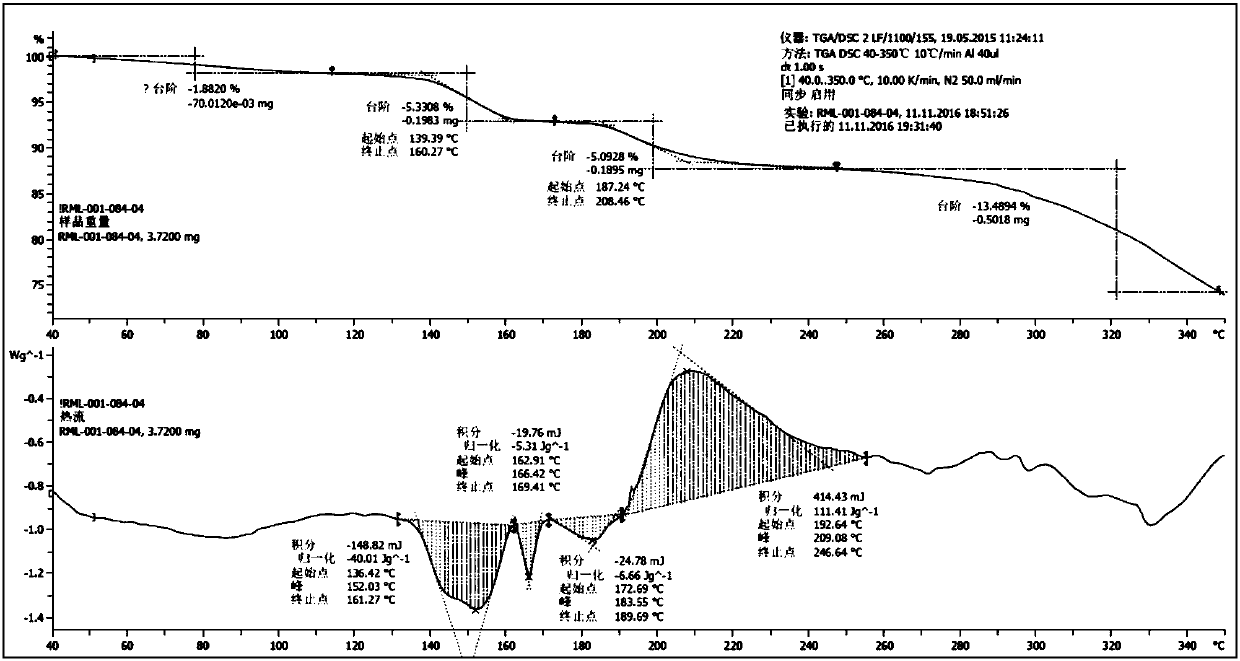
[0071] The X-ray diffraction spectrum figure of this crystal is shown in the attached figure 1 , DSC and TGA spectra see attached figure 2 , with a melting point of 163° C., defining this crystal form as the hydrobromide III crystal form of the compound of formula (I).
Embodiment 2
[0072] Embodiment 2: the preparation of the hydrobromide α crystal form of formula (I) compound
[0073] The hydrobromide III crystal form compound can undergo crystal transformation in a non-flowing gas with a certain humidity to obtain the α crystal form, especially in air with a humidity above 75%, through the mediation of the gas phase interface, the crystal form transformation occurs . The advantage of this method is that it is not mediated by solvent, no loss occurs, and no solvent residue is introduced.
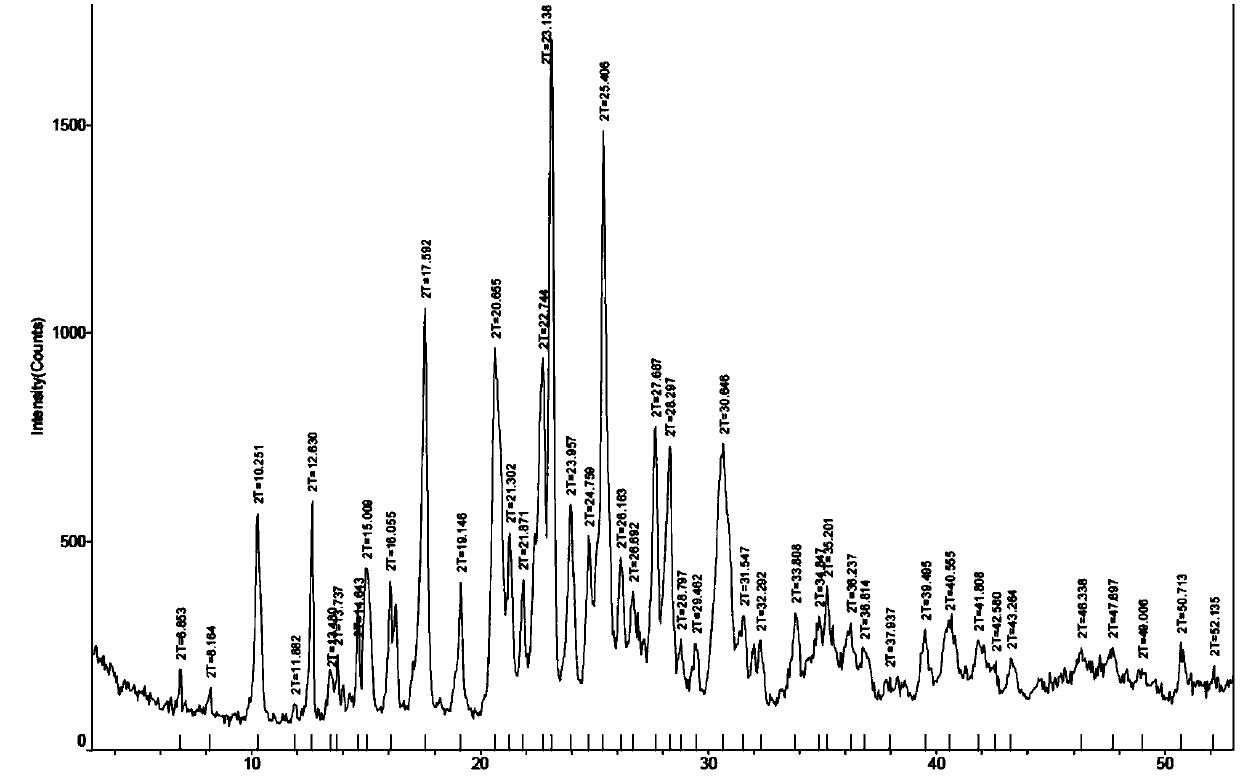
[0074] Specifically, 200 mg of the hydrobromide salt III crystal form compound of the compound of formula (I) in Example 1 was left open for 20 hours under the condition of 50-55°C-RH75%. The X-ray diffraction spectrum figure of this crystalline sample is shown in image 3 , determine that the product is formula (I ) compound hydrobromide α crystal form. IC: The content of bromide ions is 15.74%, and the ratio of hydrobromic acid to salt is confirmed to be 1:1; solv...
Embodiment 3
[0075] Embodiment 3: the preparation of the hydrobromide α crystal form of formula (I) compound
[0076] Put 500 mg of the hydrobromide III crystal form compound of the compound of formula (I) in the above Example 1, and place it under the condition of 55-60°C-RH75% for 60 hours. The X-ray diffraction spectrogram and the DSC spectrogram of the crystalline sample are studied and compared, and it is determined that the product is the hydrobromide α crystal form of the compound of formula (I).
[0077] X-ray diffraction spectrum see Figure 4 , at about 6.96, 8.24, 10.48, 12.77, 13.61, 13.85, 15.20, 16.05, 16.28, 17.70, 19.40, 20.80, 22.85, 23.23, 24.05, 24.92, 25.55, 26.25, 27.79, 28.45, 30.7 attached Figure 5 , showing a melting point of 170°C, the sample contains 4.3% free water due to no further drying after crystallization at 75% humidity, which is reflected in a weight loss platform at 60.78-79.45°C on TGA; in addition, the DVS spectrum shows a certain It has the abilit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com