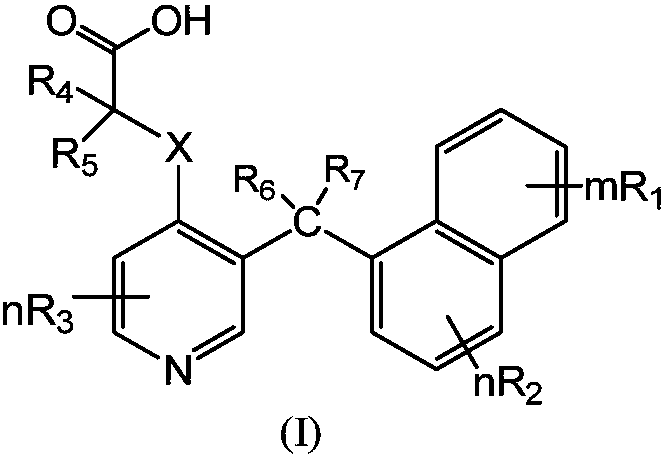
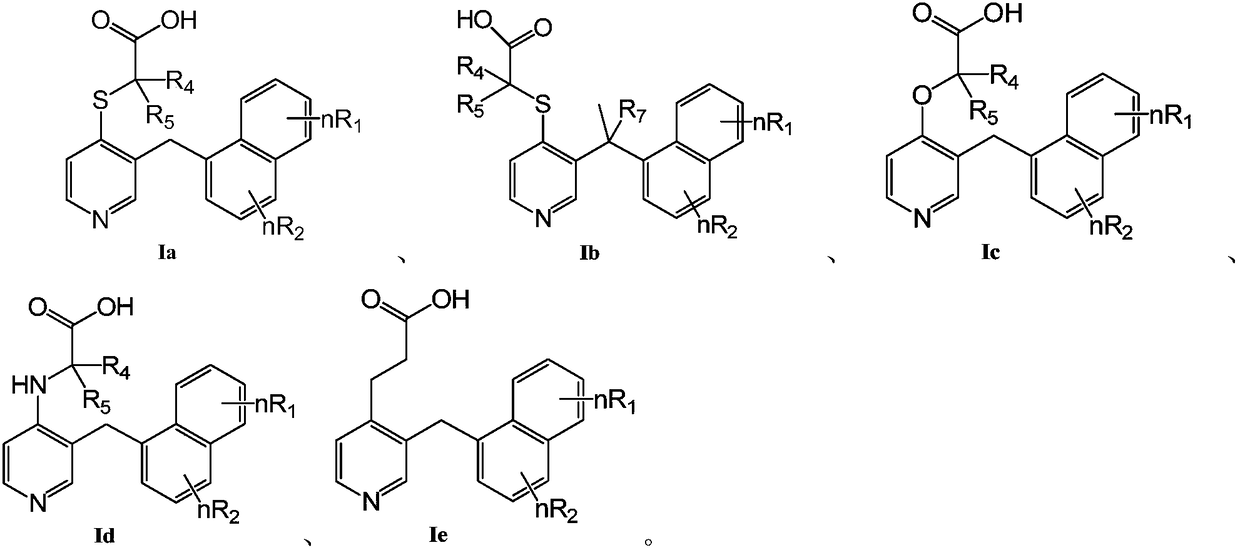
3-(naphthalene-1-methyl substituted) pyridine derivative and synthesis method and application thereof
A technology of derivatives and pyridine, applied in new compounds and their application fields, can solve problems such as no alternative drugs, low activity, and large toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
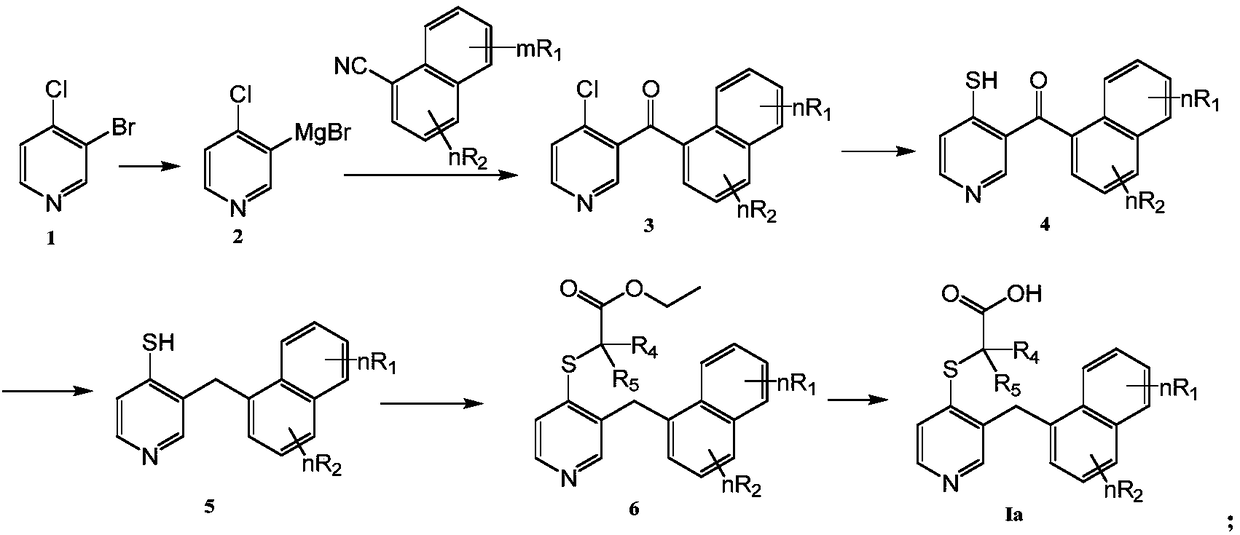
Embodiment 1
[0044] Synthesis of 2-[[3-[(4-cyanonaphthalen-1-yl)methyl]pyridin-4-yl]thio]acetic acid (Ia-1):
[0045] 1) Synthesis of 4-(4-chloronicotinoyl)-1-naphthalenecarbonitrile (compound 3a-1)
[0046]
[0047] Add magnesium chips (58mg, 2.4mmol) and catalytic amount of iodine particles in a three-necked flask equipped with a reflux condenser and a constant pressure dropping funnel, heat until the surface of the magnesium chips is purple, then add a small amount of tetrahydrofuran to cover the magnesium chips . 3-Bromo-4-chloropyridine (384mg, 2mmol) was dissolved in 5ml of tetrahydrofuran, and a small amount was added dropwise into a three-neck flask, and after being slightly heated to initiate the reaction, the remaining solution was slowly dropped into. After dropping, the reaction bottle was kept at 40° C. for 2 hours, and allowed to stand still to return to room temperature. 356mg (2mmol) of 1,4-dicyanonaphthalene and 20mL of tetrahydrofuran were cooled to 0°C, and the abov...
Embodiment 2
[0062] Synthesis of 2-[[3-[(4-fluoronaphthalen-1-yl)methyl]pyridin-4-yl]thio]acetic acid (Ia-2):
[0063]
[0064] The target compound Ia-2 was synthesized according to the synthesis method of the target compound Ia-1, and the yield was 88%.
[0065] 1 H NMR (400MHz, DMSO-d 6 )δ12.90(bs,1H),8.63(d,J=5.3Hz,1H),8.49(s,1H),8.29-8.21(m,2H),7.83-7.70(m,1H),7.63-7.57 (m,1H),7.45(d,J=7.0Hz,1H),7.40(d,J=5.3Hz,1H),7.30(d,J=7.5Hz,1H),5.70(s,2H),4.32 (s,2H).ESI-MS m / z: 326.4[M-H]-.
Embodiment 3
[0067] Synthesis of 2-[[3-[(4-chloronaphthalen-1-yl)methyl]pyridin-4-yl]thio]acetic acid (Ia-3):
[0068]
[0069] The target compound Ia-3 was synthesized according to the synthesis method of the target compound Ia-1, and the yield was 93%. 1 H NMR (400MHz,
[0070] 1 H NMR (400MHz, DMSO-d 6 )δ13.19(bs,1H),8.65(d,J=5.5Hz,1H),8.46(s,1H),8.40-8.24(m,2H),7.80-7.71(m,1H),7.65-7.56 (m,1H),7.50(d,J=7.3Hz,1H),7.43(d,J=5.4Hz,1H),7.38(d,J=8.3Hz,1H),5.62(s,2H),4.32 (s,2H).ESI-MS m / z:342.6[M-H]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com