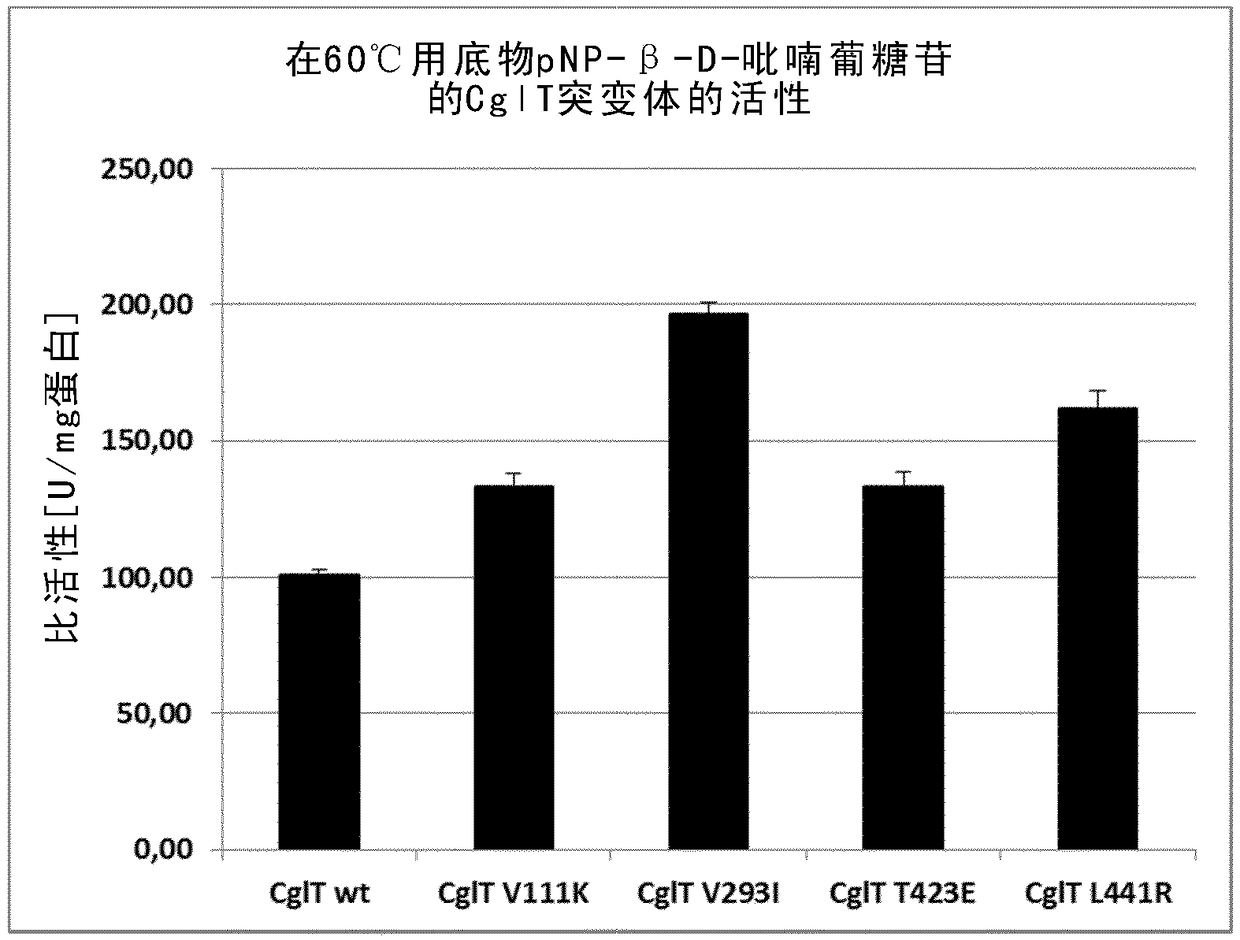
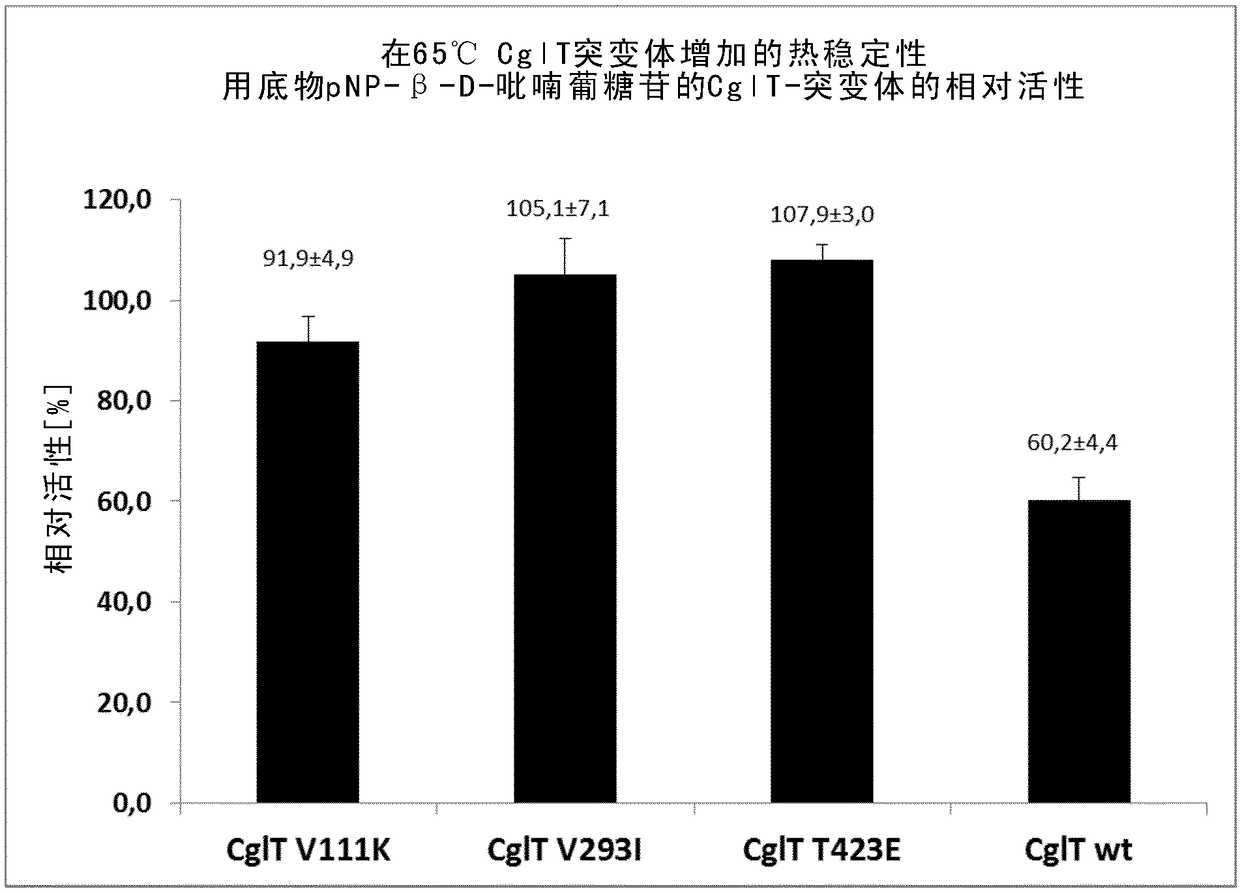
Mutant beta-glucosidase variants with increased thermostability
一种氨基酸、序列的技术,应用在糖基化酶、酶、水解酶等方向,能够解决不溶性纤维素不是等问题,达到防止终产物抑制作用、增加酶活性、改良热稳定性的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0240] Example 1: Isolation of the cglT wild-type gene
[0241] The complete gene cglT from Thermoanaerobe brucei was synthesized in codon usage in E. coli and cloned into the pET24a(+) expression vector (Novagen, Germany) fused to a C-terminal His-tag. Escherichia coli DH10B cell (Invitrogen, USA) plasmid DNA was isolated and transformed into Escherichia coli BL21Star TM (DE3) cells (Invitrogen, USA) were used for recombinant protein expression. Cells were grown in LB medium containing 100 μg / ml ampicillin (w / v) and incubated at 37°C. Liquid cultures (same medium) were shaken in a rotary shaker at 180 rpm at 37°C. LB medium contains 5 g of yeast extract, 10 g of tryptone, and 10 g of NaCl / L double distilled water; add NaOH to adjust the pH to 7.2. 16 g / l agar was added to solidify the medium.
Embodiment 2
[0242] Example 2: Mutagenesis of the cglT wild-type gene
[0243] Point mutations in the wild-type cglT gene leading to potentially stabilizing amino acid exchanges were introduced in the wild-type cglT gene by PCR site-directed mutagenesis using synthetic oligonucleotide pairs with appropriate mismatches (Table 1).
[0244] Table 1: Mutagenesis primers used
[0245] Primer
Sequence (5`→3`)
mutation
SEQ ID NO:
V111K_neu
AGCGCGATATTAAACCCGCAGCGACCATTTATC
V111K
45
V111K_rev_neu
GATAAATGGTCGCTGCGGGTTTAATATCGCGCT
46
V293I
CGATTGACTTCTTAGGCATCAATTACTACACTC
V293I
47
V293I_rev
GAGTGTAGTAATTGATGCCTAAGAAGTCAATCG
48
T423E
ATTGTGTATGTGGACTATGAGACCCAGAAACG
T423E
49
T423E_rev
CGTTTCTGGGTCTCATAGTCCACATACACAAT
50
L441R
ACAAAGAGGTGATTCGCGATGATGGGATTGAAG
L441R
51
L441R_rev
CTTCAATCCCATCATCGCGAATCACCCTTTTTGT
52
[0246] Due to ...
Embodiment 3
[0248] Example 3: Recombinant Expression of Mutant Polypeptides
[0249] Transformation of plasmids carrying wild-type and mutant β-glucosidase genes into chemically competent Escherichia coli BL21Star TM (DE3) cells for protein expression. Precultures were prepared from single colonies in liquid LB medium. After 6-8 hours of growth under aeration, expression cultures were prepared by inoculating ZYP 5052 auto-induction medium containing 2 g / l lactose and the cultures were incubated overnight [Studier, F.W., Protein production by auto-induction in high density shaking cultures . Protein Expr Purif, 2005.41:207-34.2]. Cells were harvested by centrifugation (4500 rpm, 10 min, 4°C) and cell pellets were frozen at -20°C until further use. To lyse cells, thaw cell pellets on ice, resuspend in cell lysis buffer (50mM MOPS pH 7.3, 0.5M NaCl, 20mM imidazole, 20mM CaCl 2 ) to which protease inhibitor cocktail (cOmplete, Mini, EDTA-free; pH 7.4; Hoffmann-La Roche AG, Switzerland) and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com