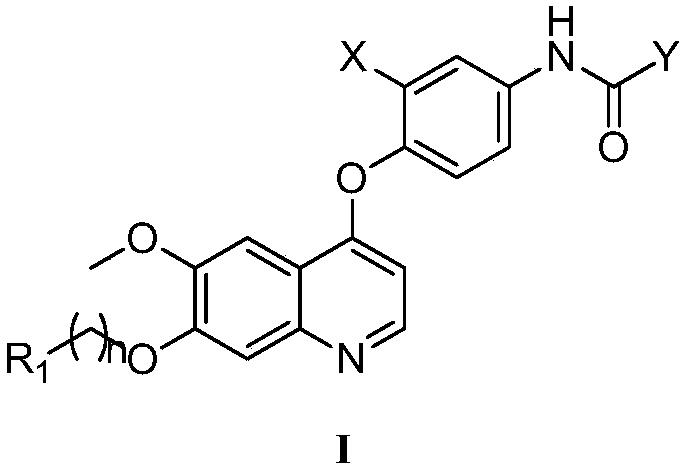
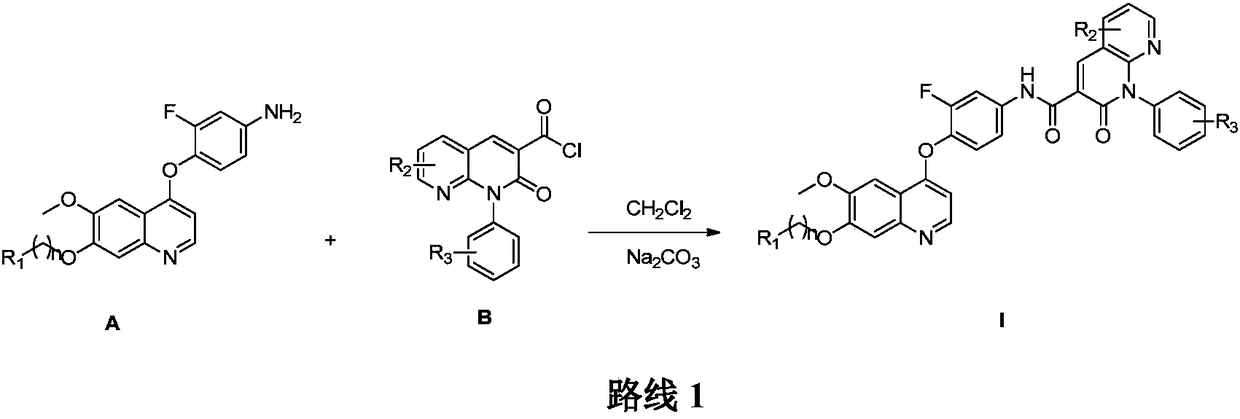
Preparation and application of 6,7-disubstituted-4-aromatic quinoline compound
A compound, quinoline technology, applied in the field of 6,7-disubstituted-4-aryl heteroquinoline compounds, can solve problems affecting tumor metastasis, neovascularization process, tumor drug resistance, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Example 1 N-(3-fluoro-4-((6-methoxy-7-(3-morpholino)quinolin-4-yl)oxy)phenyl)-1-(4-fluorobenzene base)-2-oxo-1,2-dihydro-1,8-naphthyridine-3-carboxamide
[0082] ESI-MS m / z:693.71; 1 H NMR (400MHz, DMSO-d 6 )δ11.95(s, 1H), 9.17(s, 1H), 8.63(s, 1H), 8.61(d, J=2.3Hz, 1H), 8.58(d, J=5.5Hz, 1H), 8.11( dd, J=12.9, 2.1Hz, 1H), 7.67(d, J=8.9Hz, 1H), 7.62(s, 1H), 7.51(dd, J=8.8, 3.7Hz, 2H), 7.50–7.47(m ,2H),7.46–7.42(m,2H),6.63(d,J=5.2Hz,1H),4.28(d,J=5.7Hz, 2H),3.98(s,3H),3.51(d,J= 11.8Hz, 2H), 2.99–2.87(m, 2H), 2.32(s, 2H), 1.80(d, J=13.4Hz, 2H), 1.55–1.42(m, 2H), 0.93(d, J=6.3 Hz,2H).
[0083] Step 1 Preparation of 1-(4-(3-chloropropoxy)-3-methoxy)acetophenone (II)
[0084] Add 3-methoxy-4-hydroxyacetophenone (249g, 1.5mol) and anhydrous potassium carbonate (579.6g, 2.1mol) into 1250mL acetone, control the temperature below 25°C, and slowly add 1-bromo -3-Chloropropane (661.3g, 4.2mol) / acetone (1200mL), dropwise, stirred overnight at room temperature. After the reacti...
Embodiment 2
[0113] N-(3-fluoro-4-((6-methoxy-7-(3-(4-methylpiperazin-1-yl)propoxy)quinolin-4-yl)oxy)phenyl )-1-(4-fluorophenyl)-2-oxo-1,2-dihydro-1,8-naphthyridine-3-carboxamide
[0114] ESI-MS m / z:706.27.
Embodiment 3
[0116] N-(3-fluoro-4-((6-methoxy-7-(3-(piperidin-1-yl)propoxy)quinolin-4-yl)oxy)phenyl)-1- (4-Fluorophenyl)-2-oxo-1,2-dihydro-1,8-naphthyridine-3-carboxamide
[0117] ESI-MS m / z:693.27.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com