(R)- and (S)-1-(3-(3-N,N-dimethylaminocarbonyl)phenoxyl-4-nitrophenyl)-1-ethyl-N,N'-bis (ethylene)phosphoramidate, compositions and methods for their use and preparation
A kind of technology of dimethylaminocarbonyl and aminophosphate, applied in (R)- and (S)-1-(3-(3-N,N-dimethylaminocarbonyl)phenoxy- 4-Nitrophenyl)-1-ethyl-N,N'-bis(ethylidene)amino phosphate, composition and field of use and preparation thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0088] Example 1. Preparation of compound TH 2870
[0089]
[0090] Compounds 2 to 6 were synthesized as described below.
[0091] a. Synthesis of compound 3:
[0092] in SOCl with DMF (3 drops) 2 (10 mL) to reflux compound 1 (3 g, 16.2 mmol) for 3 h and then remove SOCl under vacuum 2 . The residue was diluted with toluene (5 mL) and used in the next step without further purification.
[0093] Stir MgCl at RT 2 (930mg, 9.8mmol), TEA (4.7mL, 33.4mmol) and dimethyl malonate (1.9mL, 16.6mmol) for 1.5h, followed by the addition of the toluene solution of compound 2 mentioned above. The resulting mixture was stirred at RT for another 1.5 h, then concentrated HCl (4 mL) was added and stirred for 5 min. The mixture was extracted with EtOAc (30mL×3), dried (Na 2 SO 4 ), filtered and concentrated under reduced pressure. To the residue was added 6N HCl (30 mL) and the mixture was refluxed overnight. The mixture was extracted with EtOAc (30mL×3), dried (Na 2 SO 4 ), filte...
example 2
[0119] Example 2. Alternative preparation of compound TH 2870.
[0120]
[0121] a. Preparation of compound 3
[0122] in SOCl with DMF (10ml) 2 Compound 1 (200 g, 1.08 mol) was refluxed in (700 mL) for 3 hours and then SOCl was removed under vacuum 2 . The residue was diluted with toluene (400 mL) and used in the next step without further purification.
[0123] Stir MgCl at RT 2 (103 g, 1.08 mol), TEA (500 mL, 3.60 mol) and dimethyl malonate (145 g, 1.1 mol) for 1.5 h, then the toluene solution of compound 2 mentioned above was added dropwise. The resulting mixture was stirred for an additional 1.5 h at RT. with H 2 O (2 L) was washed, extracted with EtOAc (2 L x 5), after evaporation, 4N HCl was added until pH 6.0 and stirred for 5 min. The mixture was extracted with EtOAc (2L x 5) and evaporated.
[0124] To the residue was added 6N HCl (1500 mL) and the mixture was refluxed overnight.
[0125] The mixture was extracted with EtOAc (2 L x 5), concentrated, purifi...
example 3
[0135] Example 3. Separation of enantiomers of TH2870 by preparative chiral chromatography
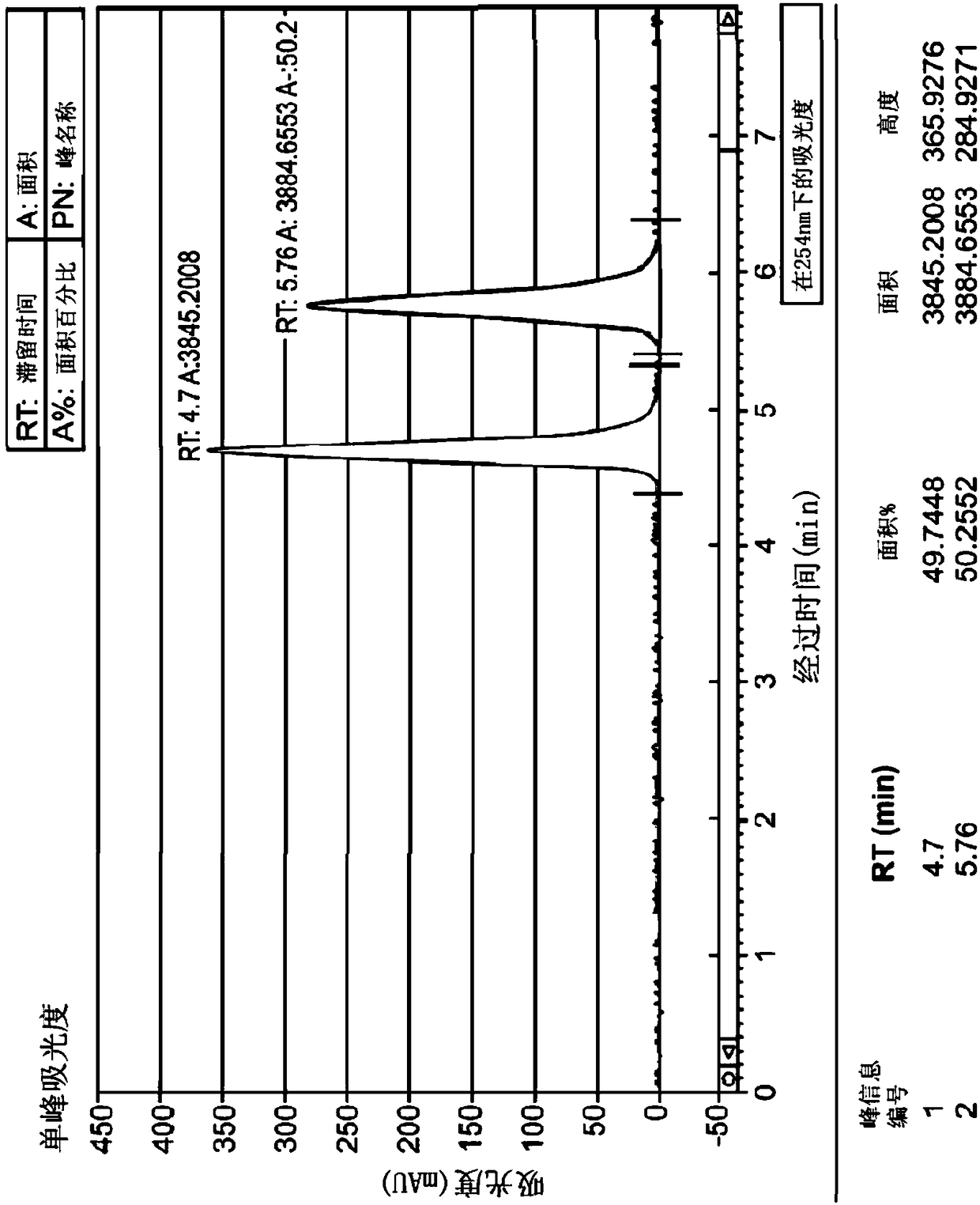
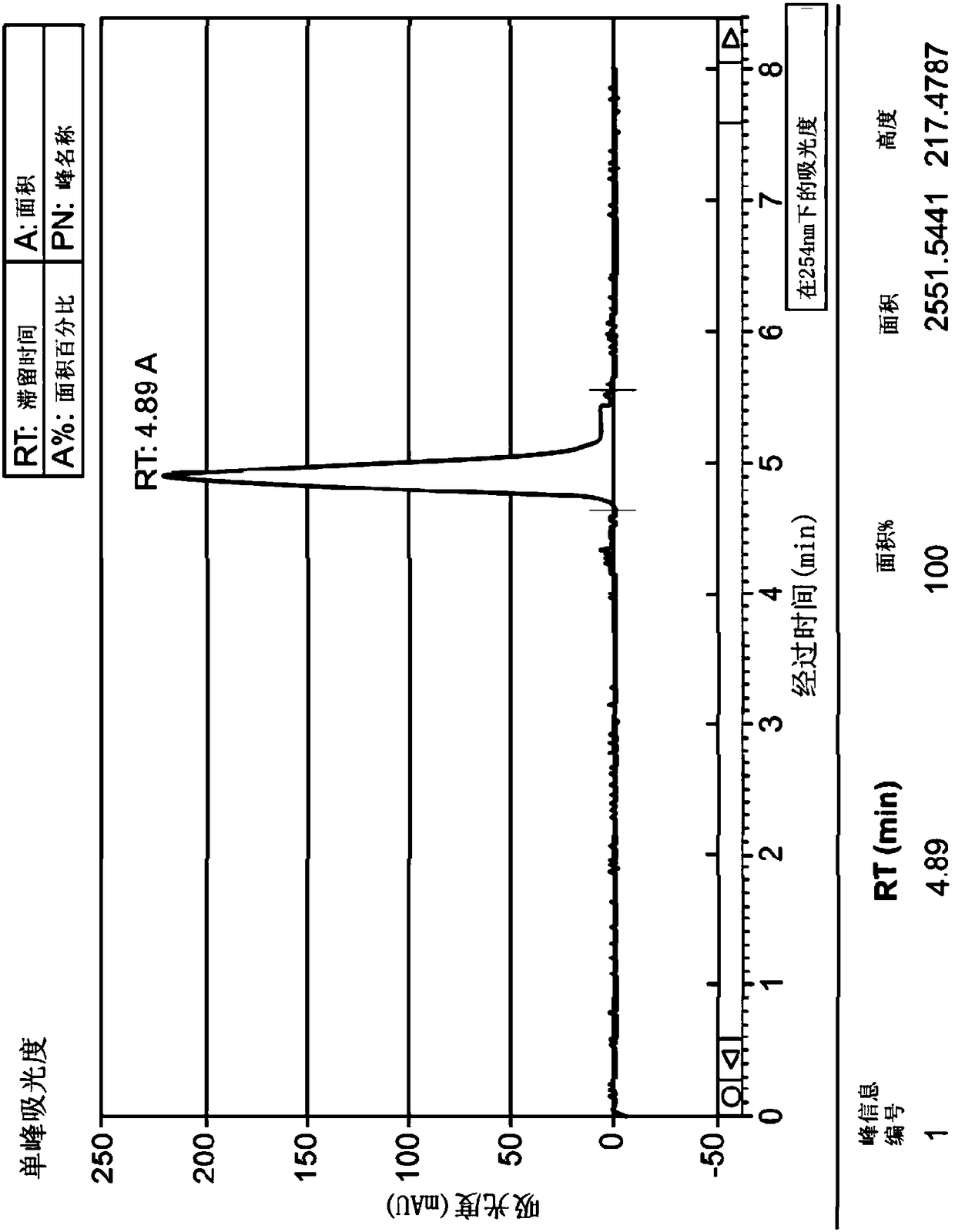
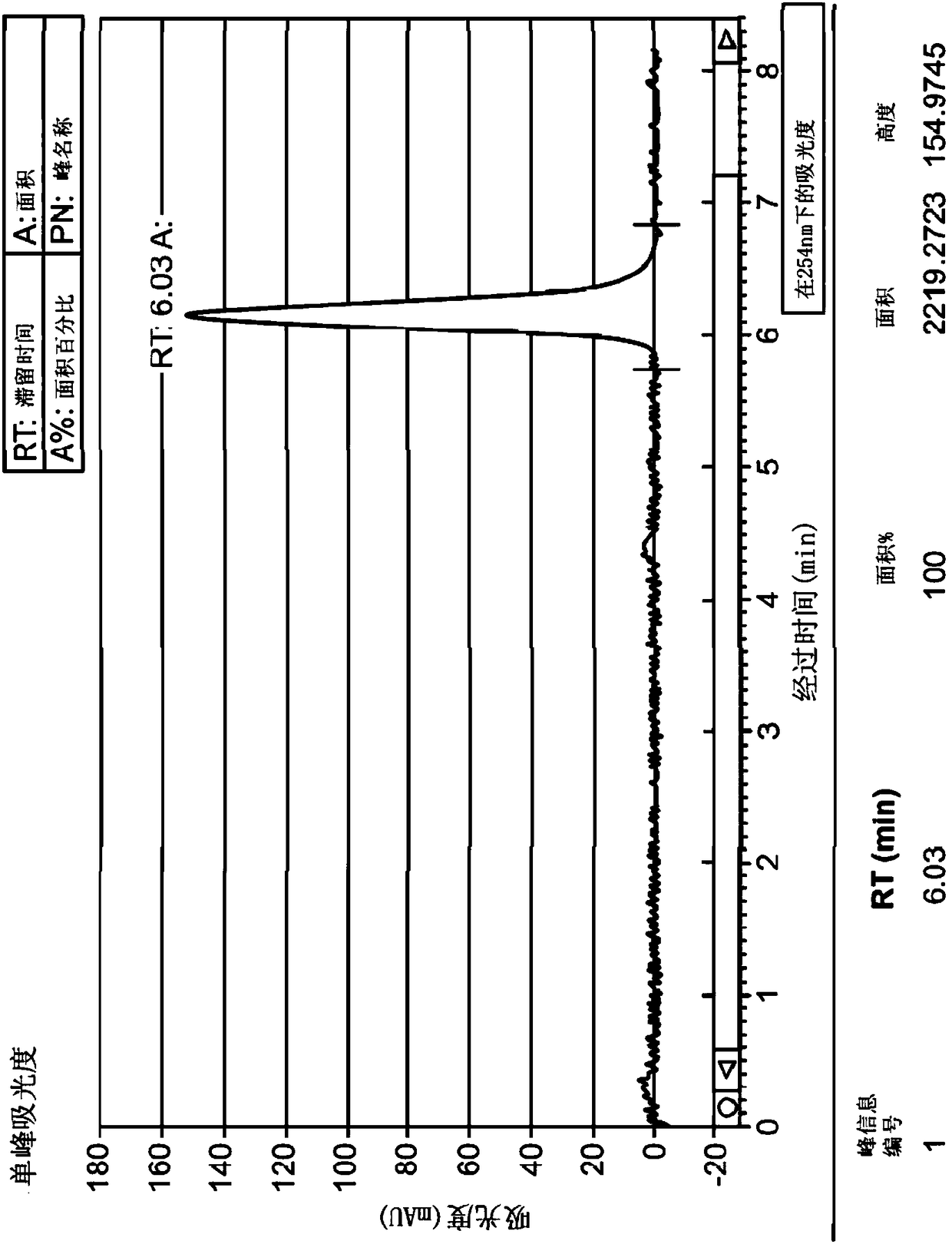
[0136] 983mg of the compound of formula (I) was dissolved in 36mL of methanol, and 1mL was injected into the SFC-80Method Station (Thar, Waters) in CHIRALPAK OZ-H 4.6 × 250mm, 5μm (Daicel), and in CO 2 Elute at this flow rate in a mixture of methanol / methanol (65-60 / 35-40). The enantiomer of formula (Ia) (configuration (R)) was obtained in 86.5% yield and 100% enantiomeric purity. The enantiomer of formula (Ib) (configuration (S)) was obtained in 83.8% yield and 100% enantiomeric purity. Figure 1 shows the purity check of TH 2870 Enantiomer 1 (TH3423) and TH 2870 Enantiomer 2 (TH3424 or AST 106) after chiral separation by LCMS.
[0137] Chiral Synthesis of TH 3423 and 3424
[0138]
[0139] Compound 2
[0140] in SOCl with DMF (2.5 mL) 2 (150 mL) was refluxed for 5 h to obtain a clear solution of compound 1 (65 g) and then the SOCl was removed under vacuum 2 . The residue wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com