Chemical self-doping of one-dimensional organic nanomaterials for high conductivity application in chemiresistive sensing gas or vapor
A resistive sensor, chemical technology, applied in the fields of organic chemistry, organic dyes, analytical materials, etc., can solve problems such as the increase in the incidence of acute respiratory diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0099] The chemical self-doping of one-dimensional organic nanomaterials has high conductivity in the chemical resistance sensing target gas or vapor application
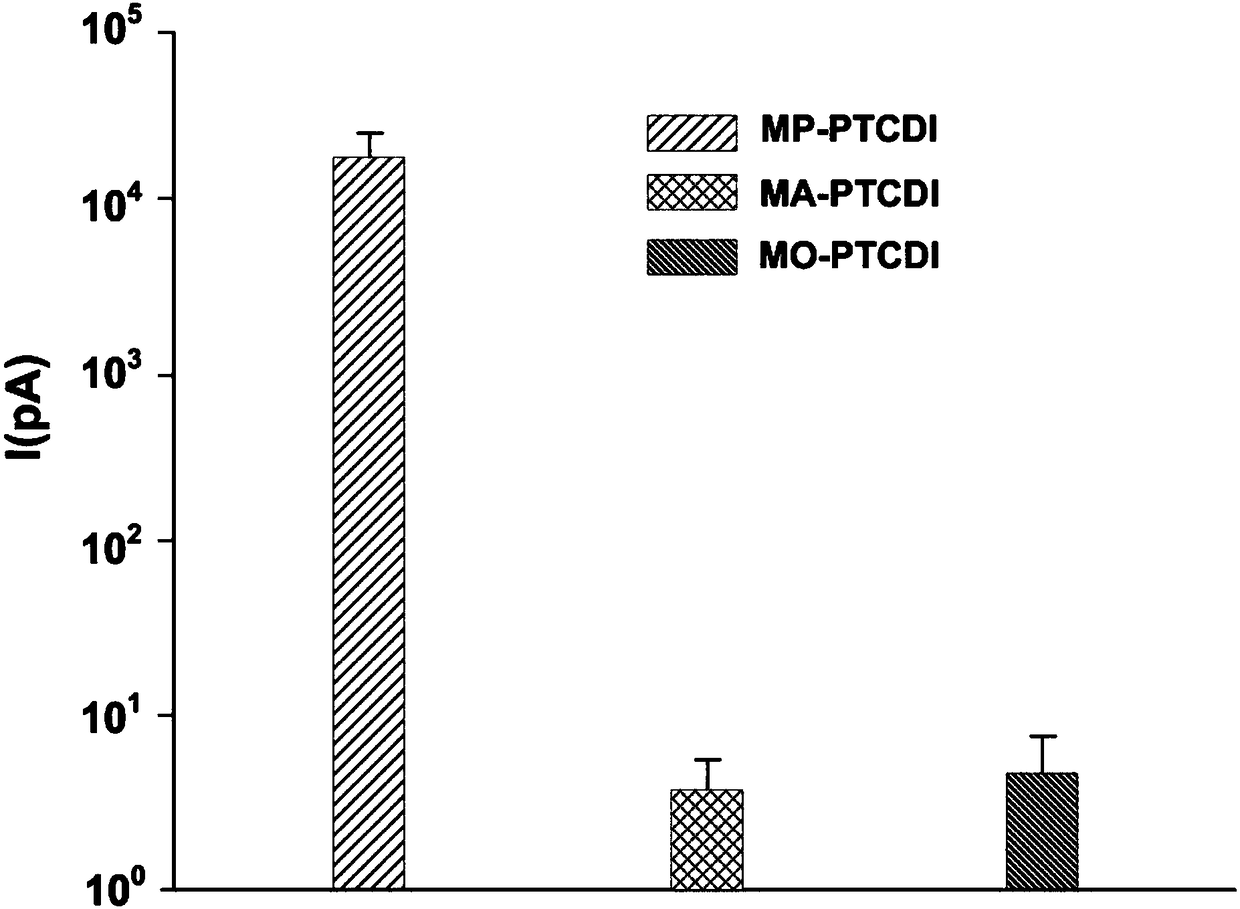
[0100] The PTCDI molecule is replaced with 1-methylpiperidine (MP) to construct a self-doped semiconductor into a nanoribbon structure through one-dimensional (1D) self-assembly of the molecule. The methylpiperidine moiety on one molecule acting as a strong electron donor (D) interacts with the PTCDI core on the adjacent molecule (acting as electron acceptor A) to generate an anionic radical of PTCDI. The resulting radicals are used as n-type dopants located in the crystal lattice of the PTCDI semiconductor. Similar self-doping induced by side groups can also be used in other conductive polymer materials such as polyaniline. The nanoribbon structure is dominated by the π-π stacking between PTCDI planes, which provides an effective way for long-distance charge transfer. Therefore, self-doped electrons migrate along ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Width | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com