Reaction Mechanism Study and Analysis Method of Aerobic Oxidation of Benzyl Alcohol to Benzaldehyde with Amphoteric Water-Soluble Catalyst
A technology of aerobic oxidation and reaction mechanism, applied in the field of computational chemistry, it can solve the problems of the design and synthesis limitation of new catalysts, the lack of progress, the inability to design new efficient catalysts to provide a theoretical basis, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0077] In order to illustrate the present invention more clearly, the present invention will be further described below in conjunction with preferred embodiments and accompanying drawings. Similar parts in the figures are denoted by the same reference numerals. Those skilled in the art should understand that the content specifically described below is illustrative rather than restrictive, and should not limit the protection scope of the present invention.
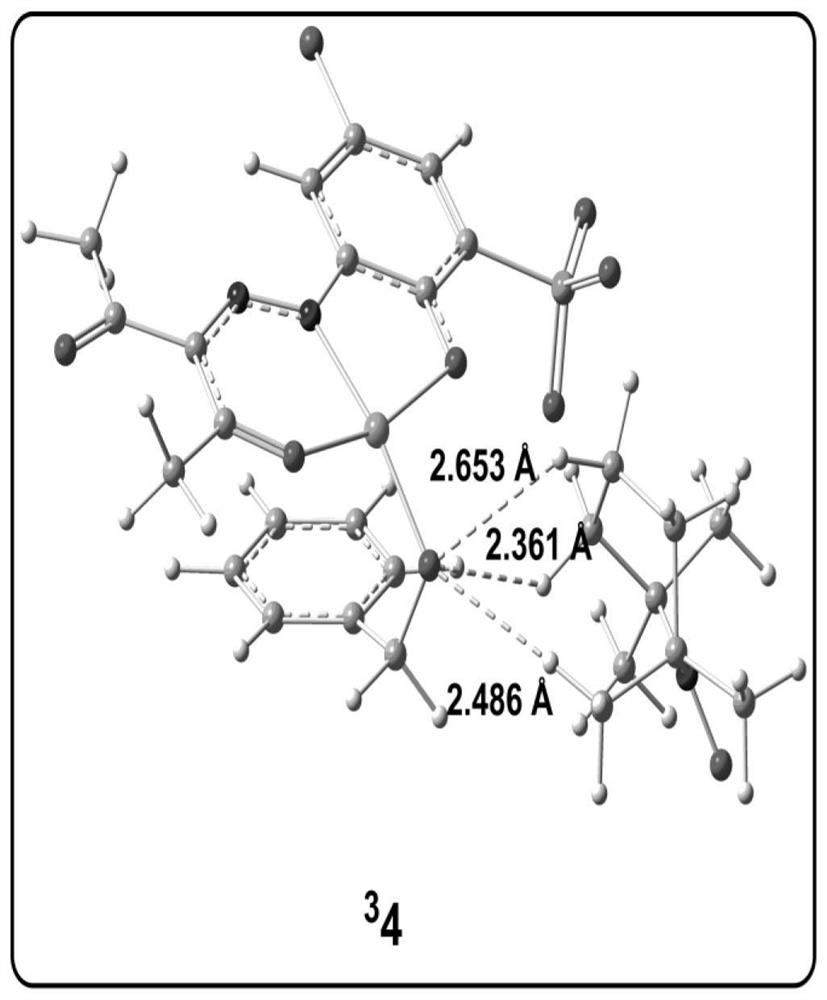
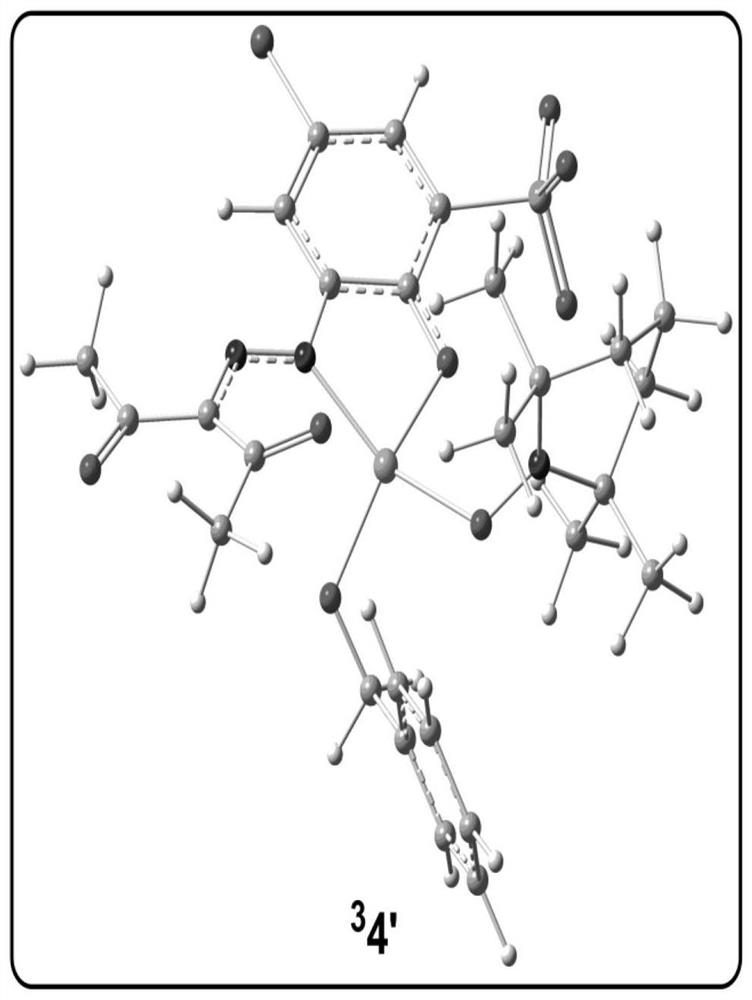
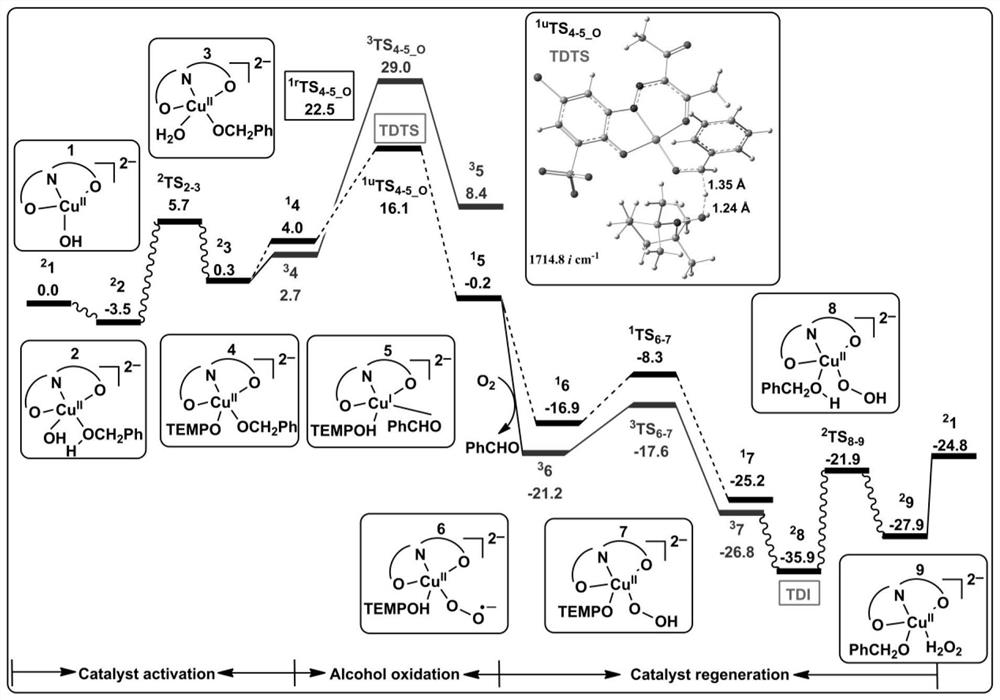
[0078] Analytical method for the study of the reaction mechanism of the aerobic oxidation of benzyl alcohol to benzaldehyde with an amphoteric water-soluble catalyst, [(L)Cu II (Hen)(H 2 O)] as shown in the following formula [compound 4 in the document Eur.J.Inorg.Chem.2011,27,4175-4181]:
[0079]
[0080] Specifically include the following steps:
[0081] (1) Construct the calculation model of the catalyst active center structure: based on the reaction condition information provided by the experiment, construct the c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com