Method for preparing high-purity rhNGF
A high-purity, cell culture technology, applied in the preparation methods of peptides, chemical instruments and methods, growth factors/inducing factors, etc., can solve the problems of high equipment requirements, unfavorable large-scale industrial amplification, and unfavorable large-scale industrial production, etc. achieve the effect of improving efficiency
Inactive Publication Date: 2018-07-03
XINTRUM PHARMA
View PDF5 Cites 8 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
The linear gradient elution method usually requires a dual-pump chromatography system, which requires high equipment and is not conducive to large-scale industrial production.
[0008] None of the above methods involve the removal of N-terminal truncation and abnormal variants, and the use of stage dynamic cleaning to remove precursor variants, and the chromatography step uses a linear gradient elution method, which is not conducive to large-scale industrial scale-up
[0009] Although each chromatography method in the prior art can remove some related impurities in rhNGF, a single method cannot satisfactorily remove all major impurities
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
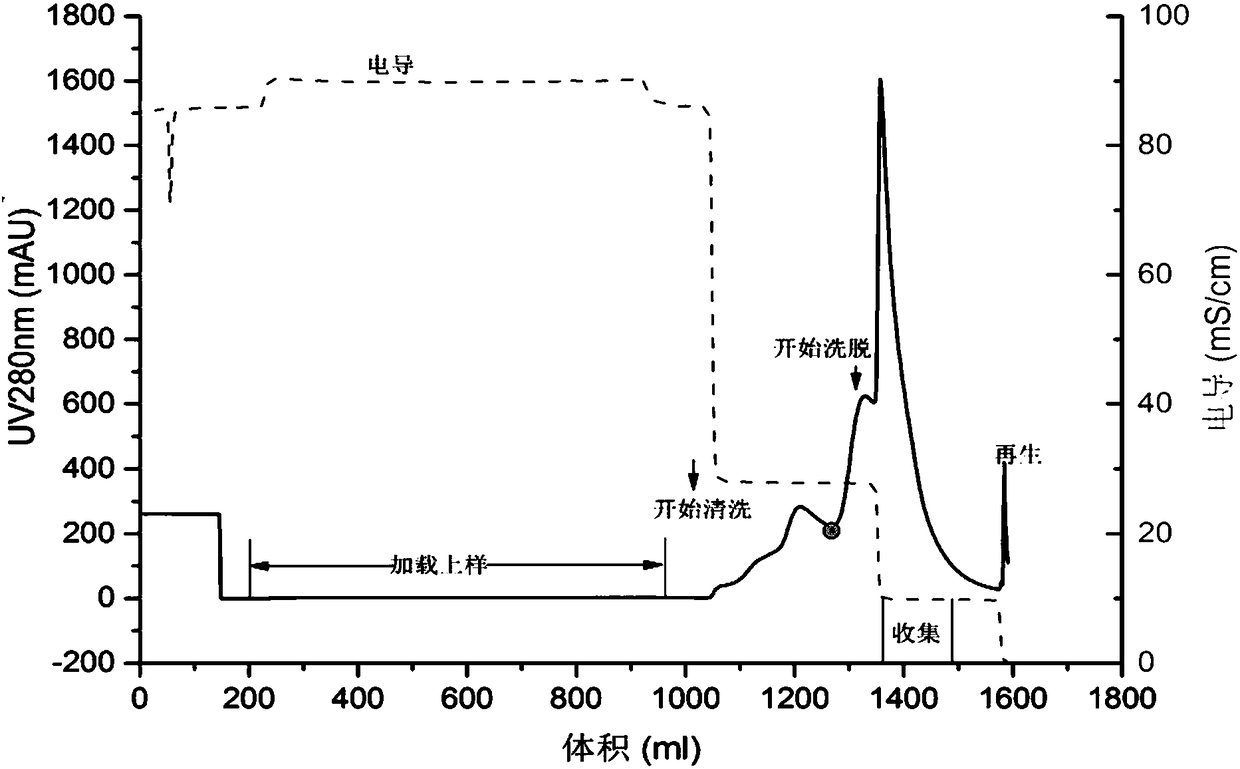
[0116] Example 1 Purification of rhNGF by Cation Exchange Chromatography and Hydrophobic Interaction Chromatography
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
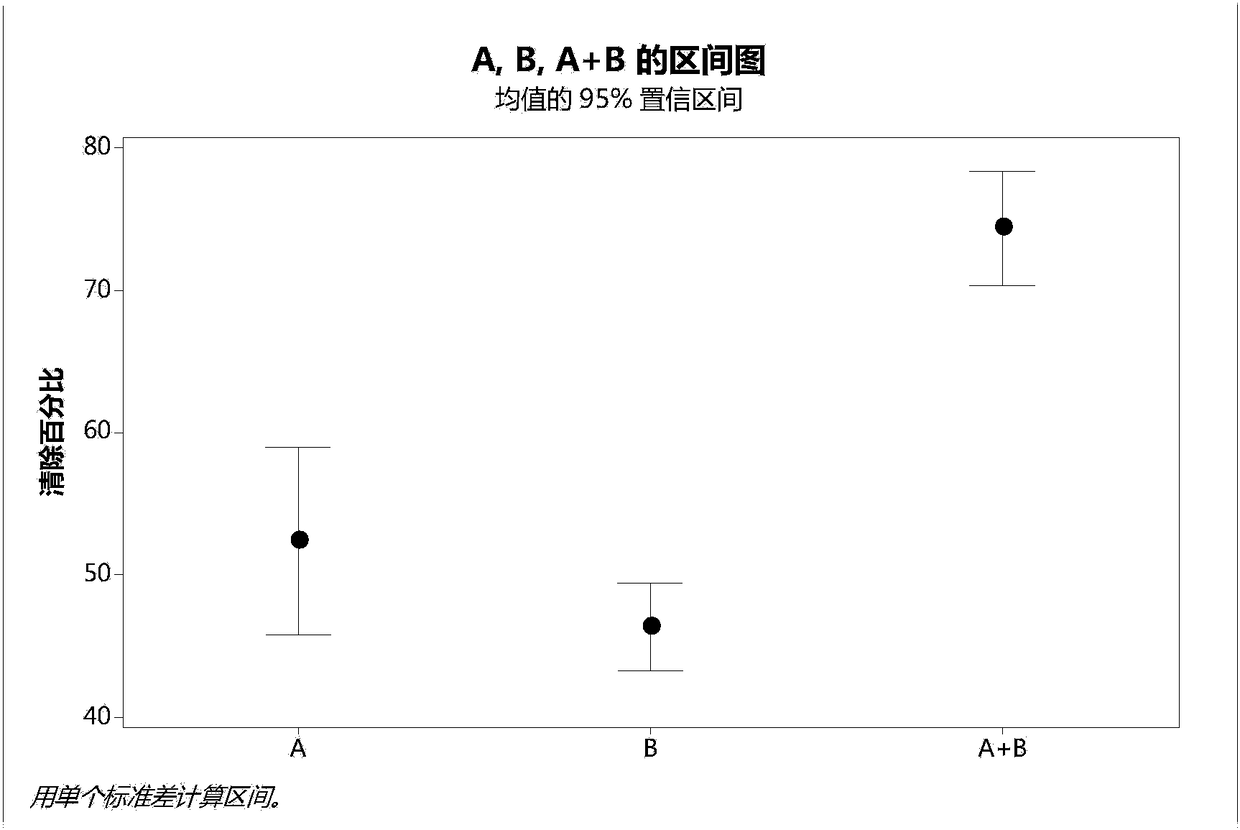
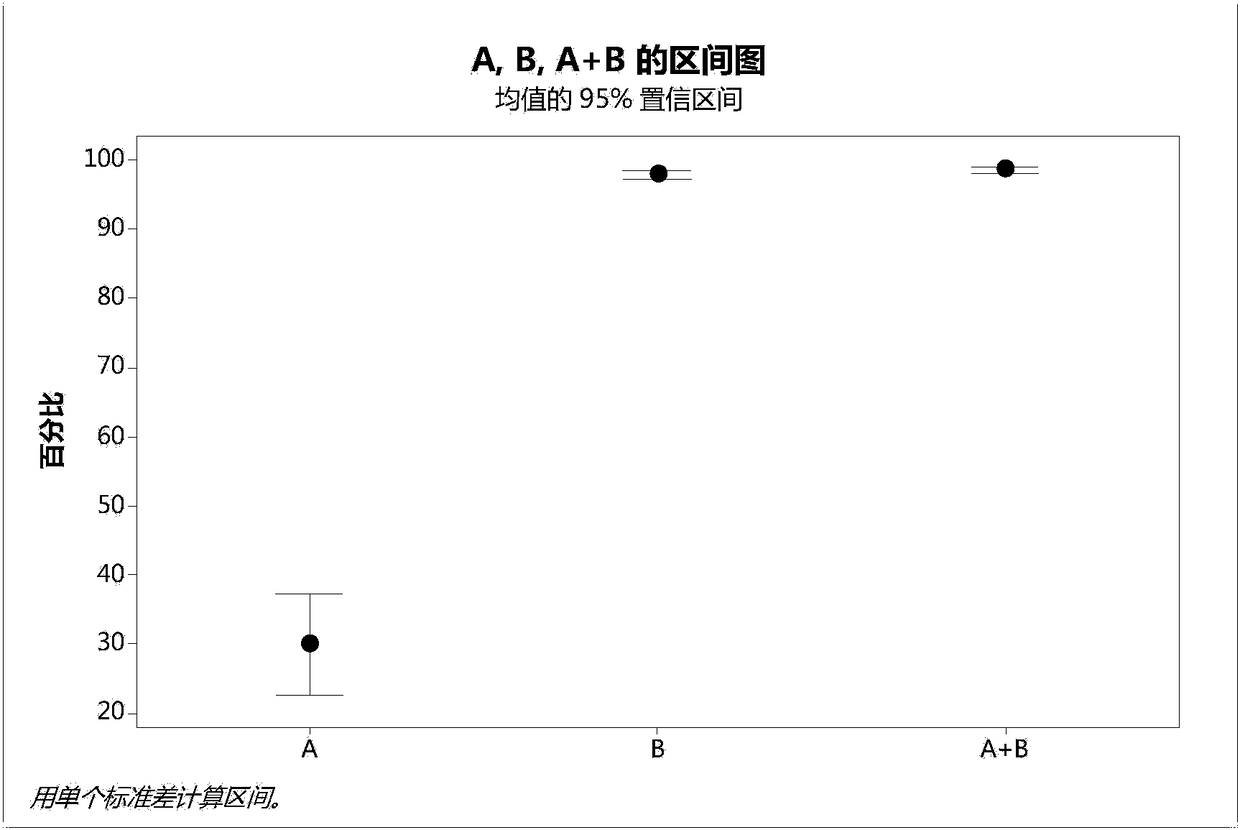
The invention provides a method for preparing high purity rhNGF from CHO cell culture, which combines cation- exchange chromatography and hydrophobic interaction chromatography. The method is characterized in that a cleaning step is added before chromatography by the two methods. Experiments prove that by the purification method, rhNGF precursor molecular variants can be removed, the content of N-terminal truncated and abnormal variants in purified products can be greatly reduced. The product has high purity and can meet the requirement of clinical application.
Description
Technical field: [0001] The invention relates to a preparation method of high-purity rhNGF, in particular to a method for obtaining high-purity rhNGF from CHO cell culture. Background technique: [0002] RhNGF expressed by genetic engineering and produced in host cells usually contains a variety of impurities, including host cell proteins, nucleic acids, and variants of the expression product rhNGF, such as precursors, N-terminal truncations, and abnormalities; Impurities of various organic and inorganic components such as endotoxins, viral contaminants, and cell culture media components will be present. Among them, N-terminal truncation and abnormal variants and precursors are the most critical impurities that affect the quality of recombinant human nerve growth factor and must be removed. [0003] The physical and chemical properties of these impurities vary, and the most effective removal methods also vary. [0004] Currently, reports on the purification of rhNGF includ...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07K14/48C07K1/20C07K1/18
CPCC07K14/48C07K1/18C07K1/20C07K1/36
Inventor 刘文超孙洪亮张怡
Owner XINTRUM PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com