Deuterated aripiprazole as well as preparation method and application thereof
A technology of aripiprazole and deuterium, which is applied in the field of deuterated aripiprazole and its preparation, can solve the problems of waste of reaction raw materials, high price, and limited wide use, and achieve saving of deuterium reagents, reasonable process design, highly operable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] A kind of preparation method of deuterated d8 aripiprazole, it comprises the following steps:
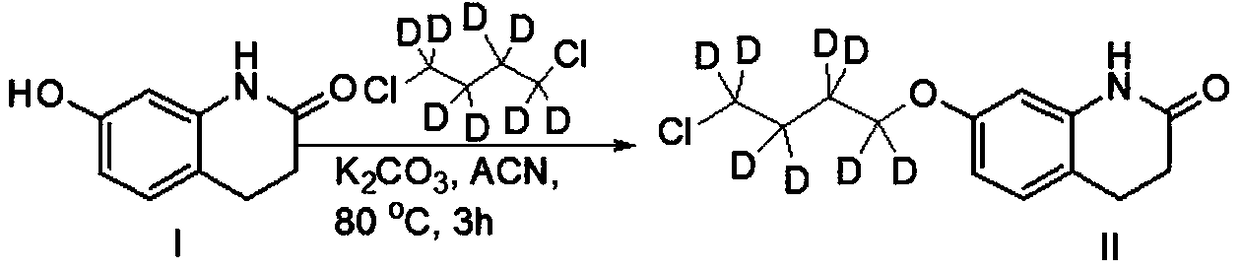
[0034] Step 1. Dissolve the raw material 3,4-dihydro-7-hydroxy-2(1H)-quinolinone (I) in a polar solvent, add a base to react for about 5 minutes, and then add 1,4-dichlorobutane Alkane-d8 (CAS: 83547-96-0), heating reaction to obtain compound (II);
[0035]
[0036] Step 2, dissolving N-Boc-piperazine (III) and 1-bromo-2,3-dichlorobenzene (IV) in a polar solvent, adding a base, and reacting by Buchwald under the catalysis of a palladium catalyst and a phosphorus catalyst Compound (IV) is obtained;
[0037]
[0038] Step 3, reacting the intermediate compound (IV) prepared in step 2 with a polar organic solvent solution of hydrochloric acid or trifluoroacetic acid to obtain the hydrochloride or trifluoroacetic acid salt of compound (V);
[0039]
[0040]Step 4, dissolving compound (V) in a polar solvent, adding base to react for 5 minutes, and then adding compound (I...
Embodiment 1
[0054] Step 1, the preparation of compound (II):
[0055]
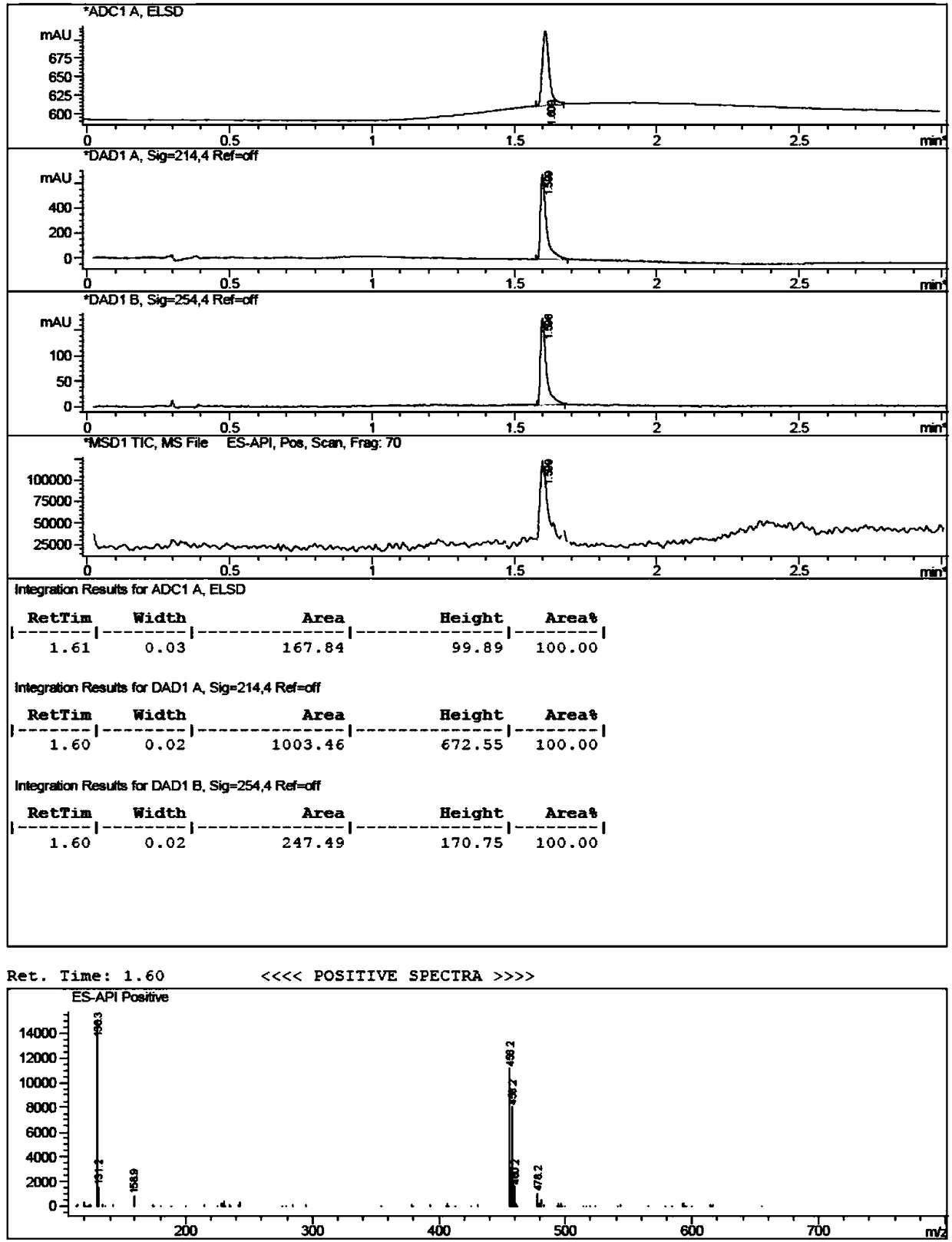
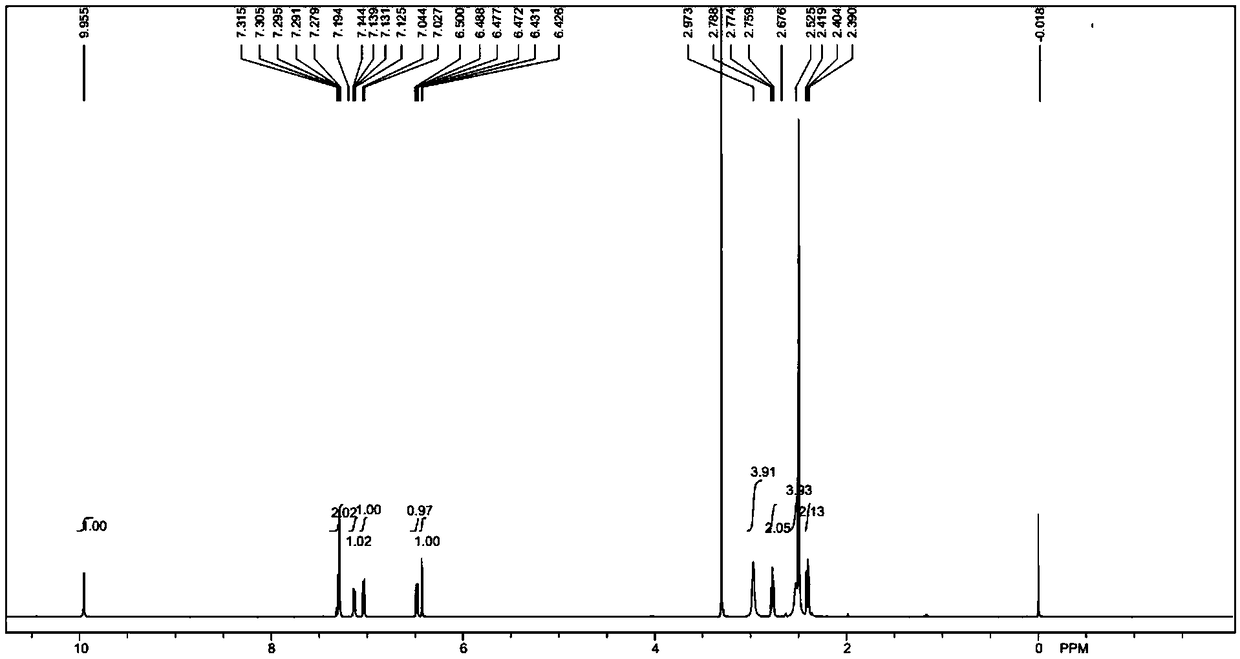
[0056] Add (1.63g, 10mmol) 3,4-dihydro-7-hydroxy-2(1H)-quinolinone, (2.48g, 18mmol) potassium carbonate, 80mL anhydrous acetonitrile into a 1000mL three-necked flask, and protect it under nitrogen The reaction was carried out for 5 minutes, and then (22.6g, 150mmol) 1,4-dichlorobutane-d8 was added, replaced by nitrogen three times, under the protection of nitrogen, the reaction was carried out at 80°C for 3 hours, TLC and LCMS monitored 3,4-dihydro- 7-Hydroxy-2(1H)-quinolinone did not remain, the reaction was basically completed, cooled to room temperature, added 30mL of water, extracted with dichloromethane (50mL×3), combined the organic phases, concentrated, and the crude product was subjected to column chromatography[ Silica gel (200-300 mesh), eluent: V (ethyl acetate): V (petroleum ether) = 6: 1] to obtain 2.40 g of light yellow solid compound (II), yield: 92.0%, LCMS [M+ H] + = 262.2.
[0057] Step 2, the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com