Method for determining conversion rate during p-polyisobutylene phenol synthesis process through quantitative nuclear magnetic resonance spectroscopy
A polyisobutylene phenol and hydrogen nuclear magnetic resonance spectroscopy technology is applied in the field of chemical analysis to achieve the effects of ensuring high efficiency, saving money and high accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] There are a batch of self-synthesized p-polyisobutylene phenol samples. According to the synthesis process estimated by the synthesizers, the conversion rate of polyisobutylene is 90%. The conversion rate of polyisobutylene needs to be determined and analyzed by hydrogen nuclear magnetic spectroscopy. The specific steps include:
[0041] Weigh 13.5067 mg of self-synthesized p-polyisobutylene phenol sample into a clean glass sample bottle, add 0.5 mL of deuterated chloroform to dissolve it and transfer it to the NMR tube. 1 H NMR test. The resonance frequency of the NMR spectrometer is 400MHz, the pulse tilt angle is 30°, D 1 It is 20s, the number of acquisition and accumulation is 32 times, the spectrum width is 12ppm, and the test temperature is 23±1℃.
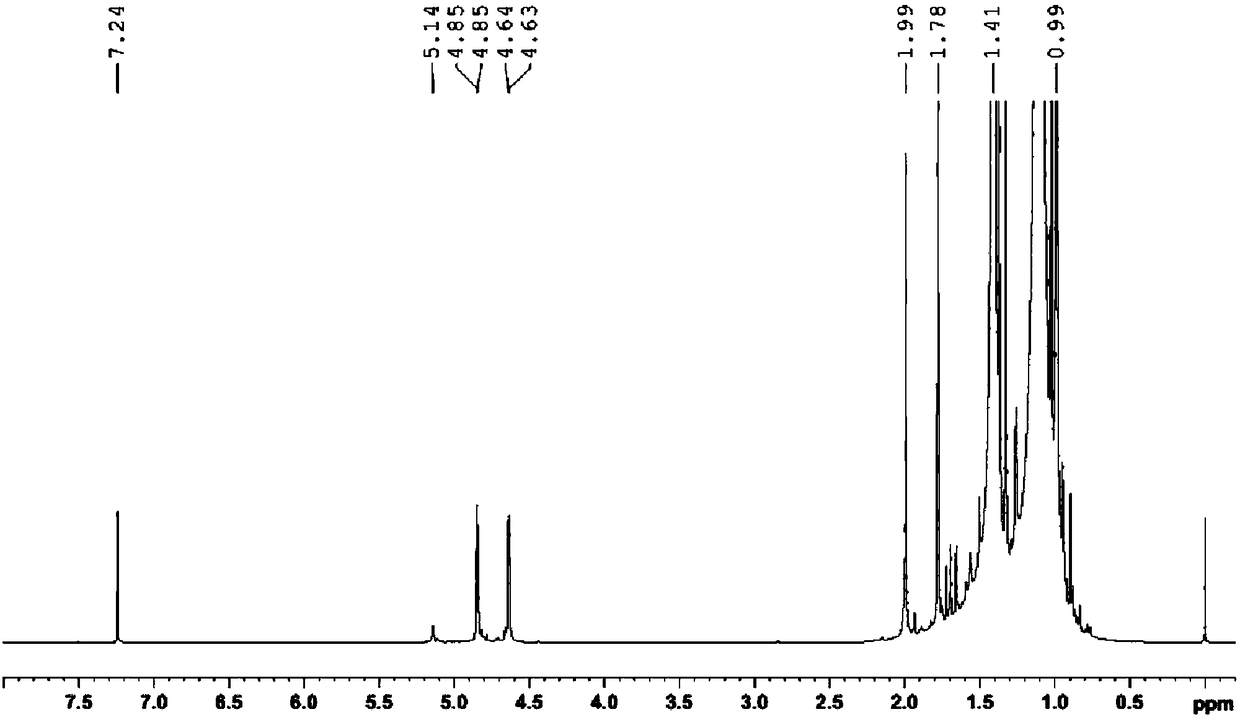
[0042] Such as Figure 4 Shown in 1 In the H NMR spectrum, A 1 = 117.70, A 2 =1.00,A 3 =(2.41+5.41), according to the conversion rate calculation formula, the conversion rate of polyisobutylene in the process of self-made p-...
Embodiment 2
[0049] There is a batch of self-synthesized polyisobutylene phenol samples. According to the synthesis process estimated by the synthesizer, the conversion rate of polyisobutylene is 80%. The conversion rate of polyisobutylene needs to be determined and analyzed by hydrogen nuclear magnetic spectroscopy. The specific steps include:
[0050] Weigh 15.5031 mg of self-synthesized p-polyisobutylene phenol sample into a clean glass sample bottle, add 0.5 mL deuterated chloroform to dissolve it and transfer it to the NMR tube. 1 H NMR test. The resonance frequency of the NMR spectrometer is 400MHz, the pulse tilt angle is 30°, D 1 It is 20s, the number of acquisition and accumulation is 32 times, the spectrum width is 12ppm, and the test temperature is 23±1℃.
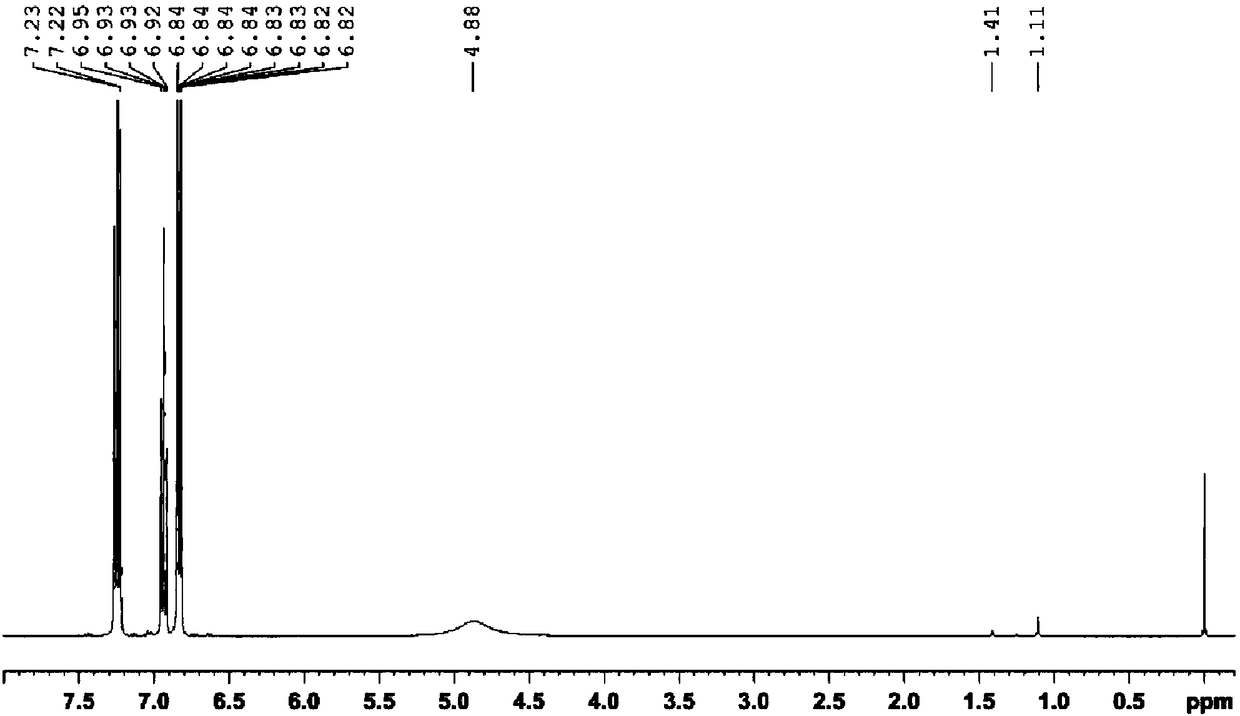
[0051] Such as Figure 5 Shown in 1 In the H NMR spectrum, A 1 = 70.88, A 2 = 1.10, A 3 =(1.56+5.89), the conversion rate of polyisobutylene in the process of self-made synthetic p-polyisobutylene phenol was calculated according to...
Embodiment 3
[0058] There is a batch of self-synthesized p-polyisobutylene phenol samples. According to the synthesis process estimated by the synthesizer, the conversion rate of polyisobutylene is 30%. The conversion rate of polyisobutylene needs to be determined and analyzed by hydrogen nuclear magnetic spectroscopy. The specific steps include:
[0059] Weigh 11.9022 mg of self-synthesized p-polyisobutylene phenol sample into a clean sample glass bottle, add 0.5 mL of deuterated chloroform to dissolve it and transfer it to a nuclear magnet tube for 1H NMR test. The resonance frequency of the NMR spectrometer is 400MHz, the pulse tilt angle is 30°, D 1 It is 20s, the number of acquisition and accumulation is 32 times, the spectrum width is 12ppm, and the test temperature is 23±1℃.
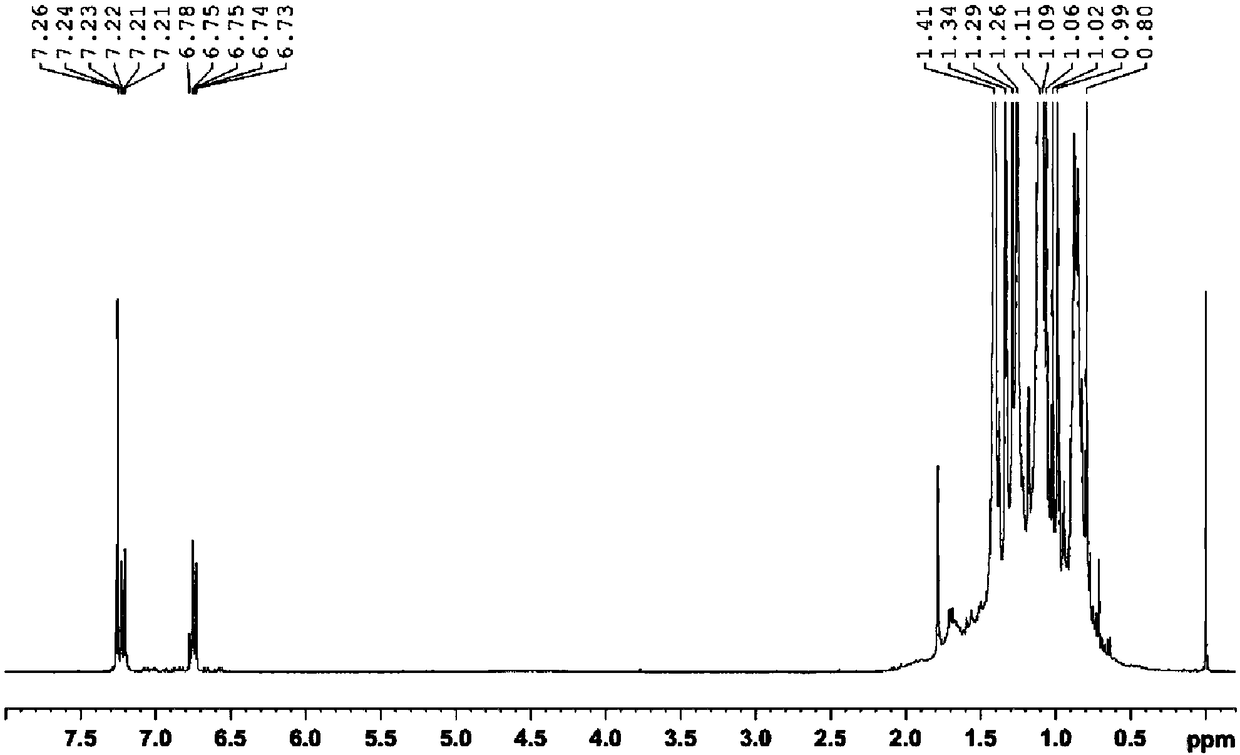
[0060] Such as Image 6 Shown in 1 In the H NMR spectrum, A 1 = 12.42, A 2 =6.43,A 3 =(3.99+3.87), the conversion rate of polyisobutylene in the process of self-made p-polyisobutylene phenol was calculated according...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Chamfer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com