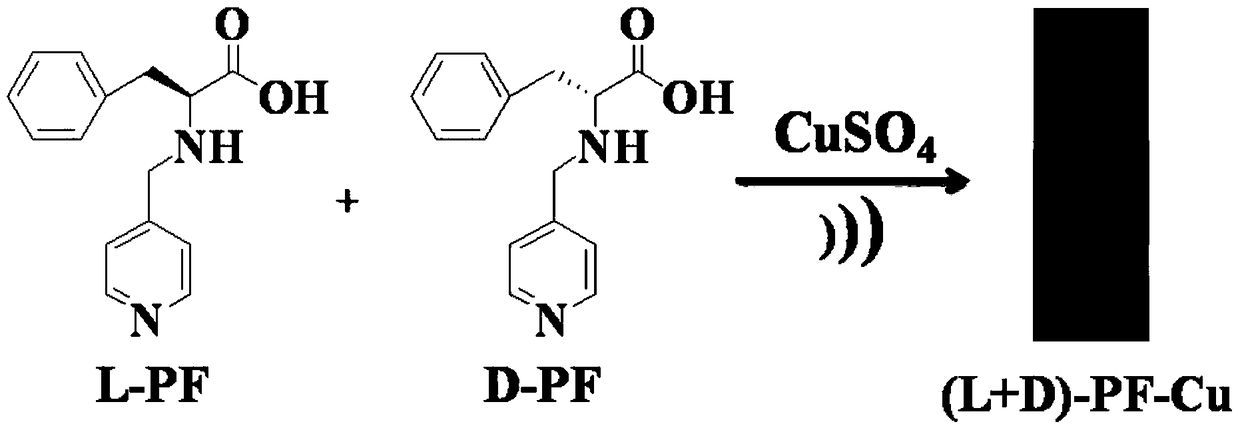
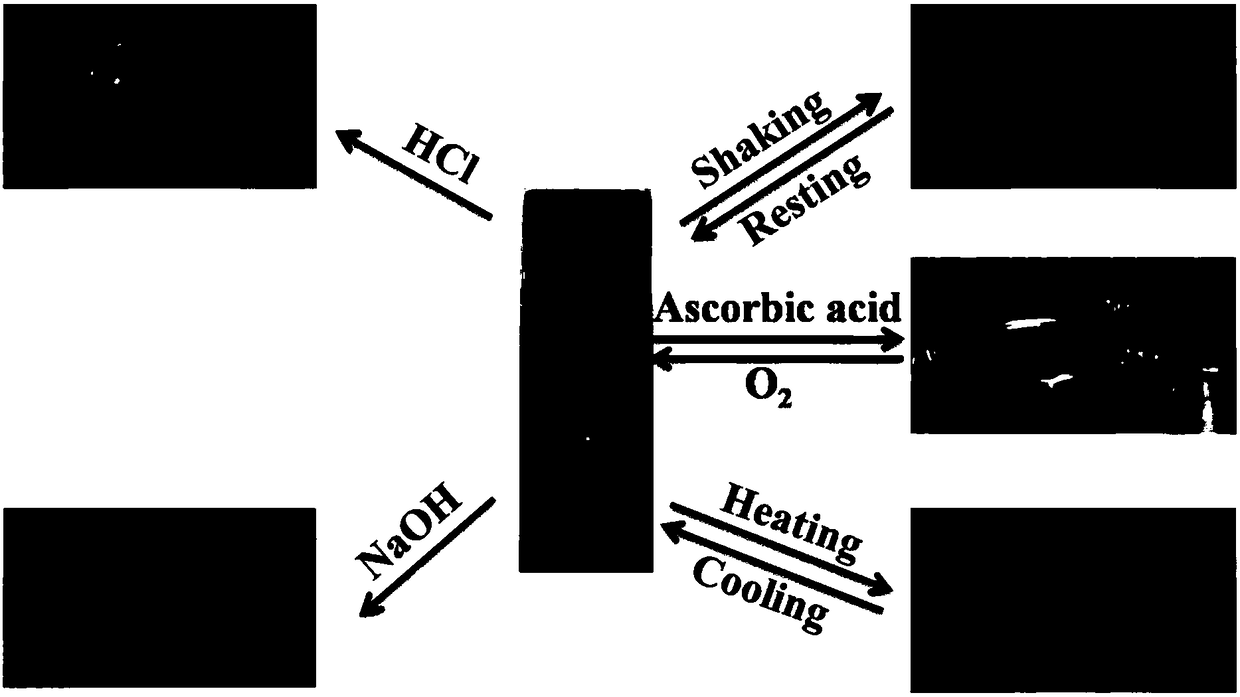
Copper (II) coordination compound, hydrogel as well as preparation method and application of copper (II) coordination compound and hydrogel
A coordination compound and hydrogel technology, applied in copper organic compounds, chemical instruments and methods, organic compound/hydride/coordination complex catalysts, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Weigh 256mg of L-PF and D-PF samples respectively to prepare 0.1M (0.1mol / L) aqueous solution of L-PF and D-PF, the solution is colorless and transparent. Prepare 0.1M CaCl separately 2 , FeCl 2 , Bi(NO 3 ) 3 , NiCl 2 , Pb(NO 3 ) 2 , CuSO 4 , La(NO 3 ) 3 , ZnCl 2 , CoCl 2 , MnSO 4 aqueous solution.
[0055] Pipette 100 μL each of 0.1M L-PF and D-PF aqueous solutions to mix, and then mix with the above-mentioned ionic solutions in a molar equivalent ratio of 2:1 in a glass bottle, adjust the pH of the solution to 7-9, and use ultrasound to observe whether A metal hydrogel is formed. It was found that in the above mixture, except for CuSO 4 In addition to the formation of hydrogels, other compounds and (L+D)-PF form precipitates or form viscous liquids or clear liquids, which cannot form metal hydrogels, as shown in the attached figure 1 shown.
[0056] (L+D)-PF aqueous solution and CuSO 4 According to the above conditions, after a few seconds of ultrason...
Embodiment 2
[0058] Prepare 0.025M aqueous solutions of L-PF and D-PF respectively, and the solutions are colorless and transparent. Prepare 0.025M CuSO 4 aqueous solution.
[0059] Pipette the above-mentioned L-PF and D-PF aqueous solutions in different ratios (10:0, 9:1, 8:2, 7:3, 6:4, 5:5, 4:6, 3:7, 2 :8,1:9,0:10) were mixed to form a (L+D)-PF solution, and then each (L+D)-PF was mixed with an equal concentration of CuSO 4 The solution was mixed in a glass bottle according to 2:1 molar equivalent, the pH of the solution was adjusted to 7-9, and ultrasonication was performed. As a result, it was found that the above-mentioned mixture L-PF and D-PF had different equivalent ratios, and different colors would appear. Although metal hydrogels could not be formed (the concentrations of L-PF and D-PF were lower than the gel-forming concentration), L-PF and D-PF When the D-PF equivalent ratio is 1:1, it will still appear purple, as attached Figure 6 shown.
Embodiment 3
[0061] Prepare 0.005M aqueous solutions of L-PF and D-PF respectively, and the solutions are colorless and transparent. Prepare 0.005M CuSO 4 aqueous solution.
[0062] Pipette 0.005M L-PF and D-PF aqueous solutions in different ratios (10:0, 9:1, 8:2, 7:3, 6:4, 5:5, 4:6, 3:7, 2:8, 1:9, 0:10) to form a (L+D)-PF solution, and then each (L+D)-PF was mixed with an equal concentration of Cu(II) solution at a molar equivalent of 2:1 to adjust The pH of the solution is 7-9, ultrasonication, the color change of each system is the same as that of Example 2, but the color is slightly lighter.
[0063] Measure the EPR spectrum of the above-mentioned (L+D)-PF-Cu respectively, as attached Figure 7 shown. It was found that the mixing ratio of L-PF and D-PF was from 10:0 to 5:5, the Cu(Ⅱ) signal gradually weakened, and the signal of oxygen anions gradually increased; the mixing ratio of L-PF and D-PF was from 5:5 to At 0:10, the Cu(Ⅱ) signal gradually increased, and the oxygen anion s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com