Chiral helicene with binaphthol structure and preparation method of chiral helicene
A technology of binaphthol and chirality, which is applied in the field of design and synthesis of chiral catalysts, can solve the problems of complex and expensive synthetic routes of precursor compounds, unfavorable preparation and application of chiral helicene, etc., and achieve good asymmetric catalysis or non- Symmetrical recognition effect, correct structure, and short synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
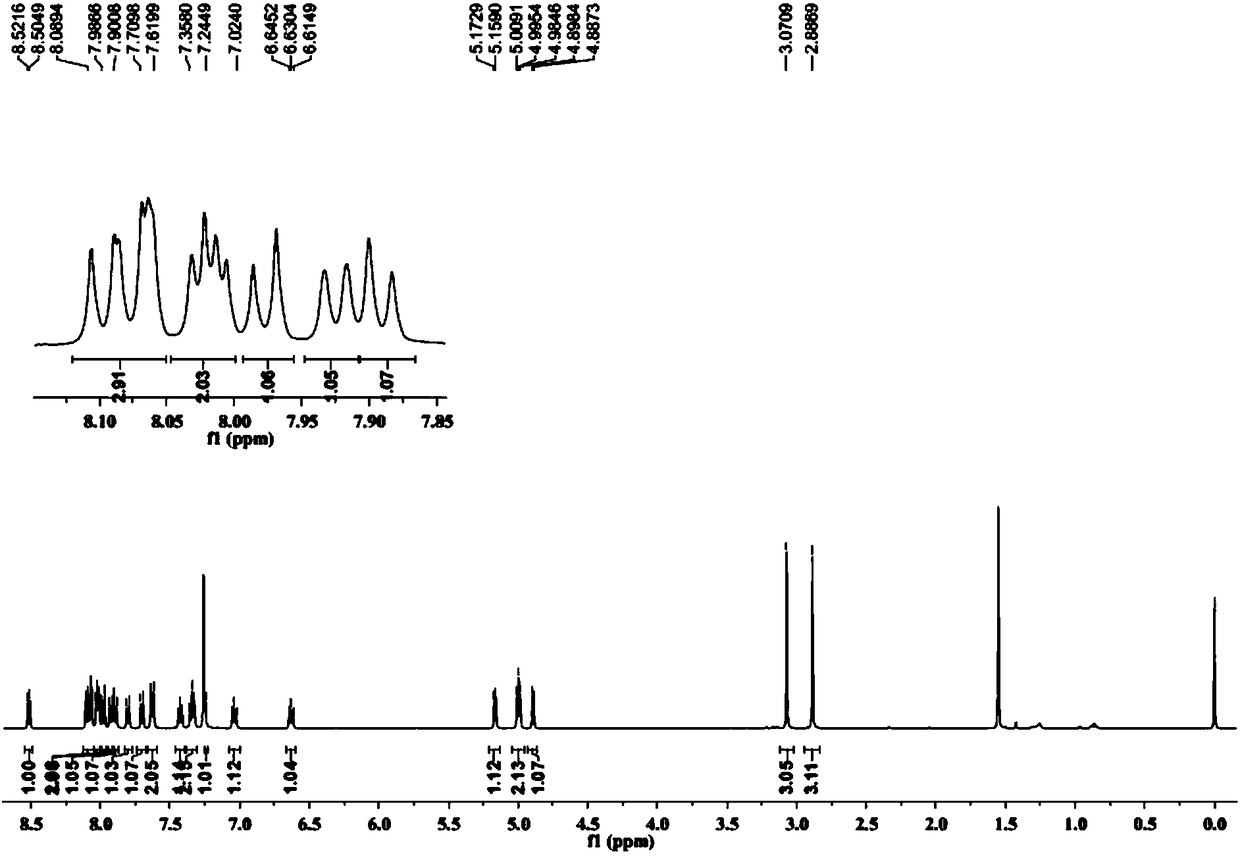
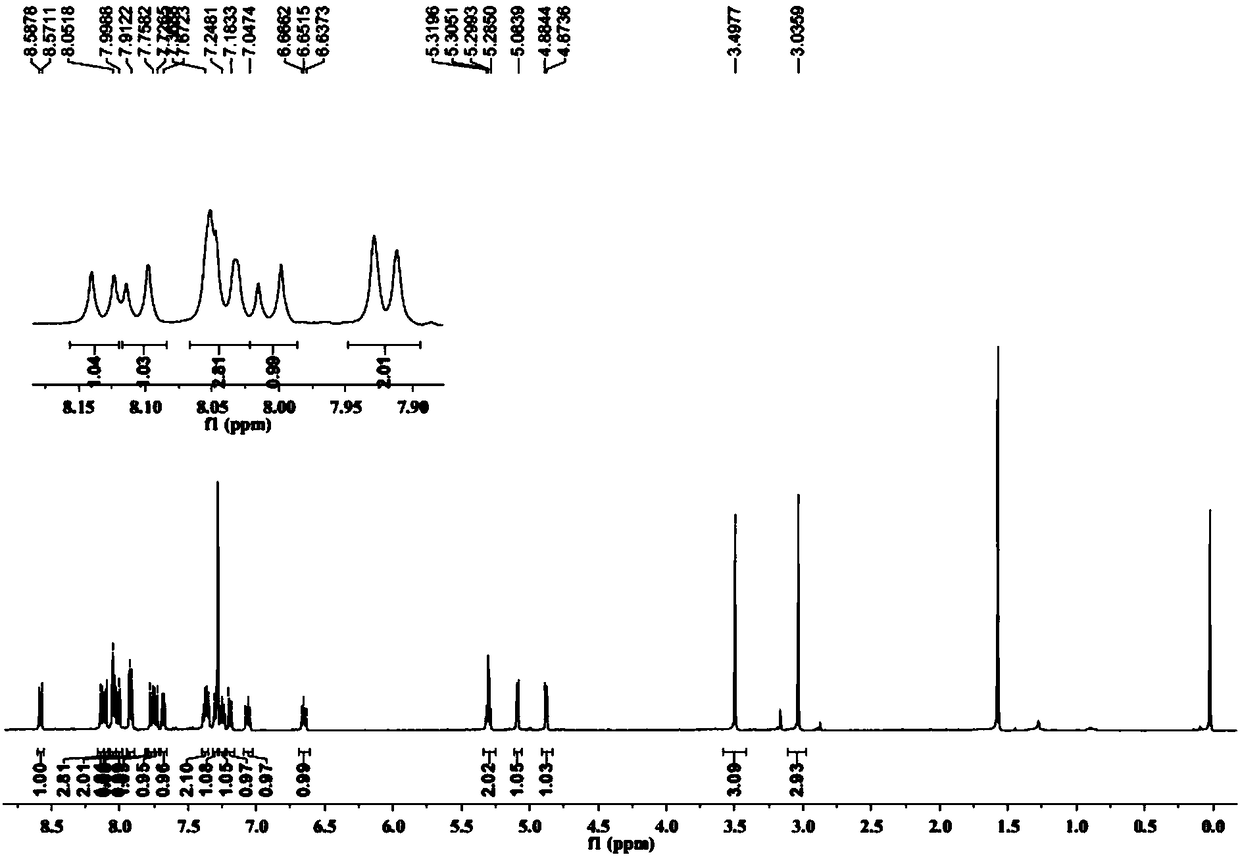
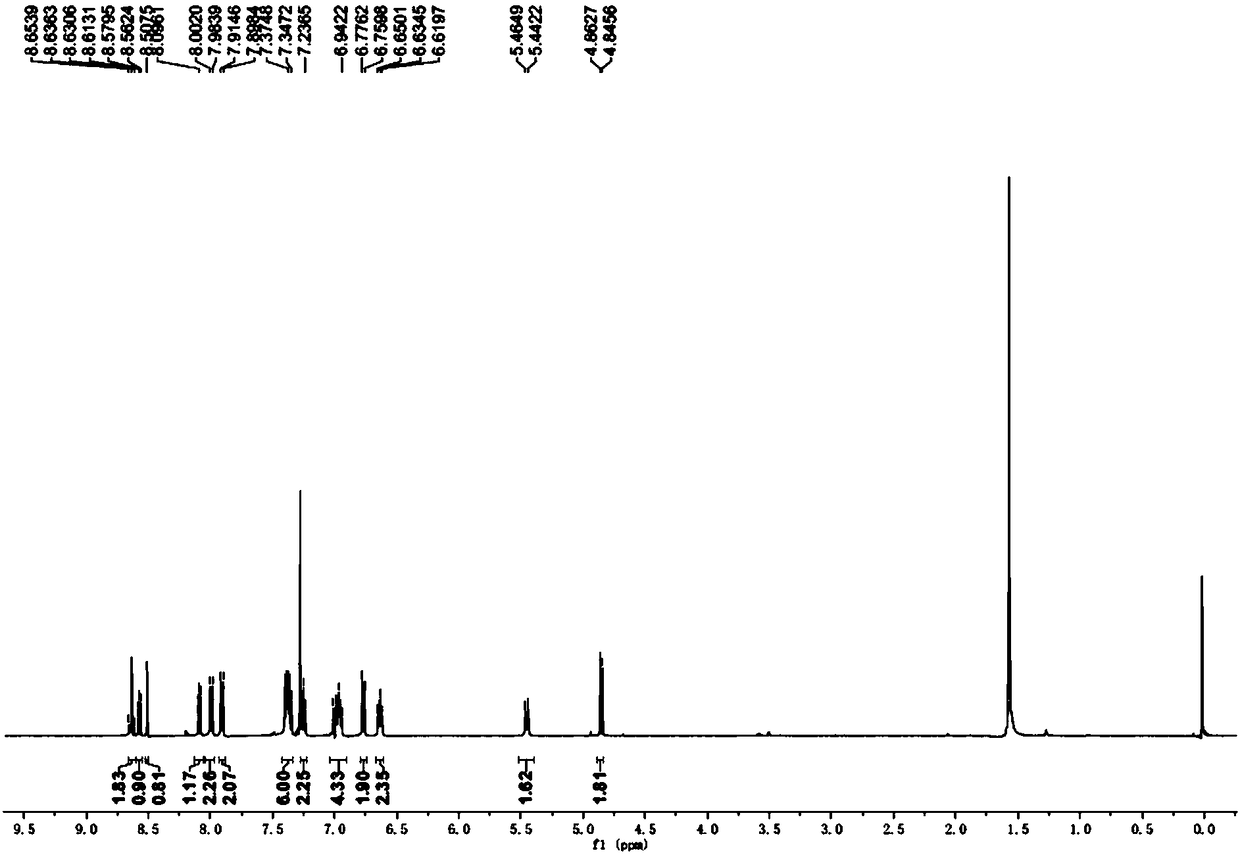
Image
Examples
Embodiment 1
[0030] Example 1: Preparation of 5-(2-(methoxymethoxy)naphthalene-1-yl)-6-methoxymethoxy-15-bromo[6]helicene
[0031] a. Synthesis of intermediate 3-(4-(4-bromostyryl) styryl)-2,2'-bis(methoxymethoxy)-1,1'-binaphthalene
[0032]
[0033] 0.2g (R)-2,2'-bis(methoxymethoxy)-[1,1'-binaphthyl]-3-aldehyde and 0.32g (E)-(4-(4-bromobenzene Vinyl)benzyl)triphenylphosphine bromide (1.05eq.) was added to 15mL of dry THF, and sodium tert-butoxide (1.5eq.) was added in batches under ice-bath conditions, and the solution turned orange-red. Stir in an ice bath, and after half an hour, stir at room temperature until the reaction is complete. 20 mL of water was added to the reaction solution to quench the reaction, the aqueous phase was extracted with 20 mL of dichloromethane, the organic phase was washed with 20 mL of saturated brine, dried over anhydrous sodium sulfate, and the solvent was spun off to obtain a yellow solid with a yield of 89%.
[0034] b, the synthesis of target 5-(2-(m...
Embodiment 2
[0051] Example 2: 6,13-bis(methoxymethoxy)-5,14-bis(2-(methoxymethoxy)naphthalen-1-yl)-9-bromo-[7]helicene preparation of
[0052] a. Intermediate 1,4-bis(2-(2,2'-bis(methoxymethoxy)-[1,1'-binaphthyl]-3-yl)vinyl)-2-bromobenzene Synthesis
[0053]
[0054] 0.3g (R)-2,2'-bis(methoxymethoxy)-[1,1'-binaphthyl]-3-aldehyde and 0.35g ((2-bromo-1,4-phenylene (yl)bis(methylene))bistriphenylphosphine bromide (0.55 eq.) was added to 15 mL of dry THF. Under ice-cooling, sodium tert-butoxide (2.5eq.) was added in batches, and the solution turned orange-red. Under ice bath, stir for half an hour, rise to room temperature and stir until the reaction is complete. 20 mL of water was added to the reaction solution to quench the reaction, the aqueous phase was extracted with 20 mL of dichloromethane, the organic phase was washed with 20 mL of saturated brine, dried over anhydrous sodium sulfate, and the solvent was spun off to obtain a yellow solid with a yield of 91%.
[0055] b. Synthe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com