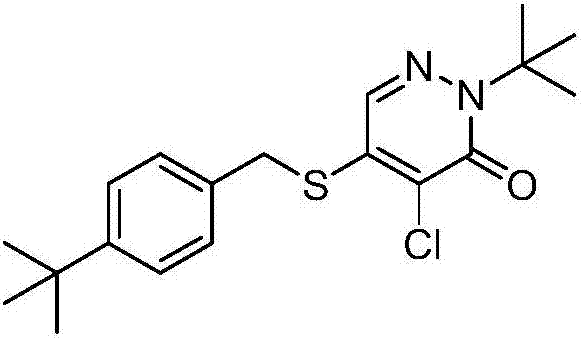
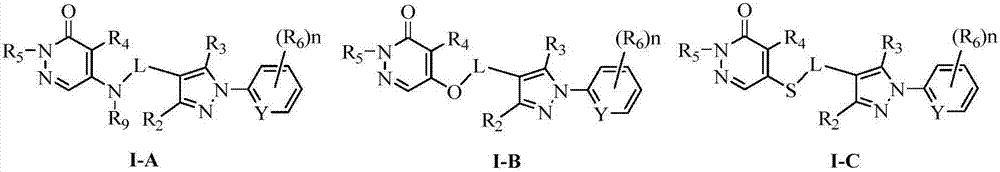
Pyridazinone compound and application thereof
A kind of compound, technology of pyridazinone, applied in pyridazinone compound and its application field, can solve the problem that structure pyridazinone compound has not been reported etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0481] Example 1: Preparation of Compounds 15-21
[0482] 1) Preparation of 1-(2,4-dichlorophenyl)-1H-pyrazole
[0483] Take 21.35g (0.1mol) of 2,4-dichlorophenylhydrazine hydrochloride and 16.4g (0.1mol) of 1,1,3,3-tetramethoxypropane in a 250ml three-necked flask, 100ml of 95% ethanol aqueous solution As a solvent, the temperature was raised to reflux for 3-5 hours. After the completion of the reaction monitored by TLC, most of the ethanol was evaporated under reduced pressure, sodium carbonate aqueous solution was added to the residual liquid, the aqueous phase was extracted with (3×100 ml) ethyl acetate, the organic phases were combined, dried over anhydrous magnesium sulfate, filtered, and desolvated . The residue was separated by column chromatography (the eluent was ethyl acetate and petroleum ether, the volume ratio was 1:10) to obtain 18.06 g of a yellow solid with a yield of 84.8%.
[0484] 2) Preparation of 1-(2,4-dichlorophenyl)-1H-pyrazole-4-carbaldehyde
[04...
Embodiment 2
[0496] Example 2: Preparation of compound 74-1
[0497] 1) 2.89 g (0.02 mol) of phenylhydrazine hydrochloride and 3.28 g (0.02 mol) of 1,1,3,3-tetramethoxypropane were added to 50 ml of 95% ethanol. The temperature was raised to 80°C and refluxed, and the reaction was carried out for 3-6 hours. After TLC monitoring was completed, the solvent was evaporated under reduced pressure, and (3×50ml) ethyl acetate was added for extraction. The organic phase was washed with saturated brine 50ml. Chromatography (eluent is ethyl acetate and petroleum ether (boiling range 60-90° C.), volume ratio is 1:6) to obtain 2.45 g of oily liquid with a yield of 85.0%.
[0498] 2) 7.3g (0.1mol) DMF was added to the there-necked flask of 250ml, after stirring in an ice-salt bath, 15.4g (0.1mol) phosphorus oxychloride was added dropwise, and 2.88g (0.02mol) N- Phenylpyrazole was heated to 100°C and refluxed, and reacted for 3-6 hours. After TLC monitoring was completed, the reaction solution was pour...
Embodiment 3
[0504] Example 3: Preparation of compound 124-3
[0505] 1) 4.29 g (0.02 mol) of 4,6-dichloropyridylhydrazine hydrochloride and 3.28 g (0.02 mol) of 1,1,3,3-tetramethoxypropane were added to 50 ml of 95% ethanol. The temperature was raised to 80°C and refluxed, and the reaction was carried out for 3-6 hours. After TLC monitoring was completed, the solvent was evaporated under reduced pressure, and (3×50ml) ethyl acetate was added for extraction. The organic phase was washed with saturated brine 50ml. Chromatography (the eluent is ethyl acetate and petroleum ether (boiling range 60-90° C.), the volume ratio is 1:6), to obtain 3.51 g of oily liquid with a yield of 82.0%.
[0506] 2) 7.3g (0.1mol) DMF was added to the there-necked flask of 250ml, after stirring under the ice-salt bath, 15.4g (0.1mol) phosphorus oxychloride was added dropwise, and 4.28g (0.02mol) 4.28g (0.02mol) was added dropwise after 1 h, 6-dichloropyridine-1H-pyrazole was heated to 100°C and refluxed, and rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com