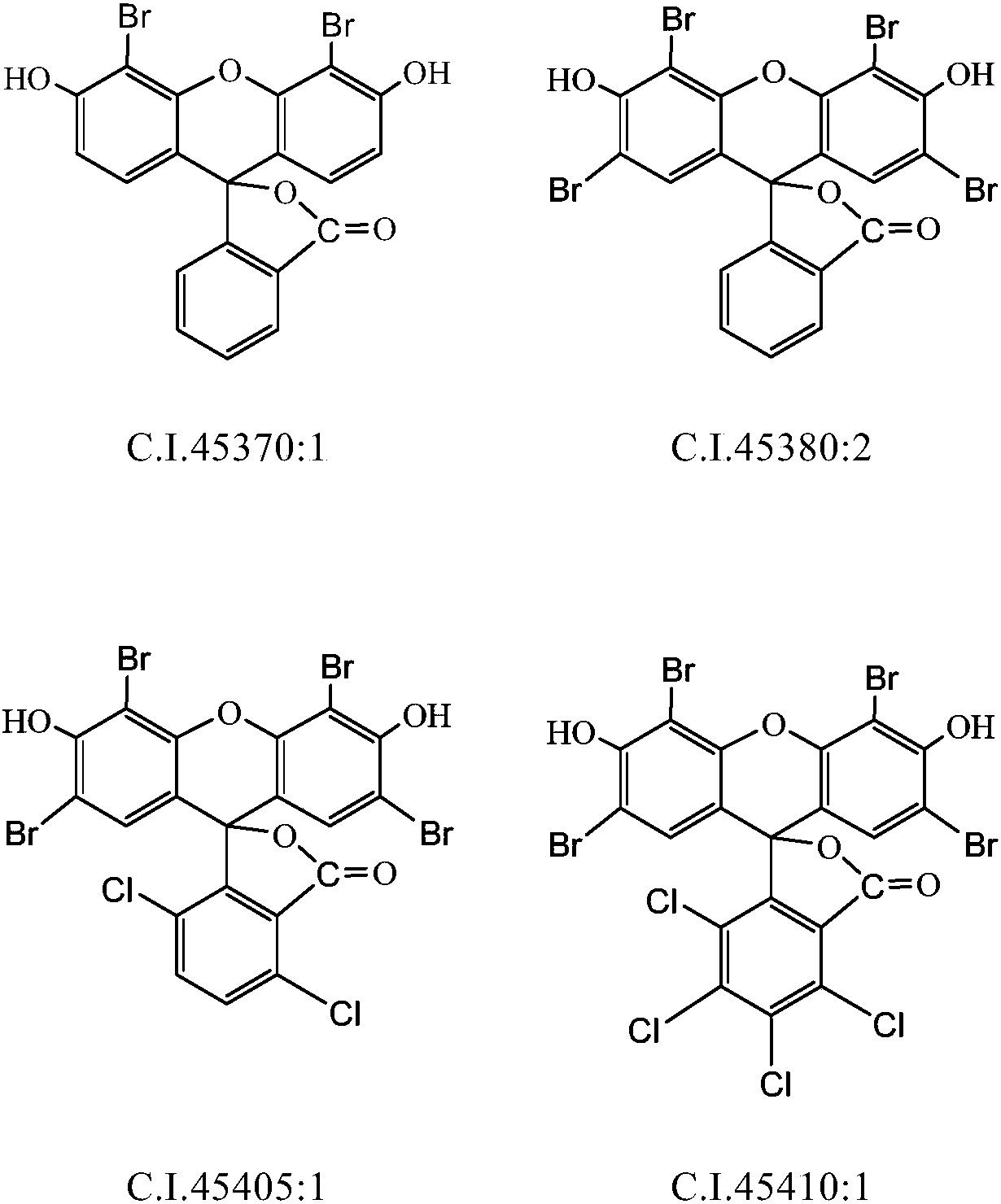
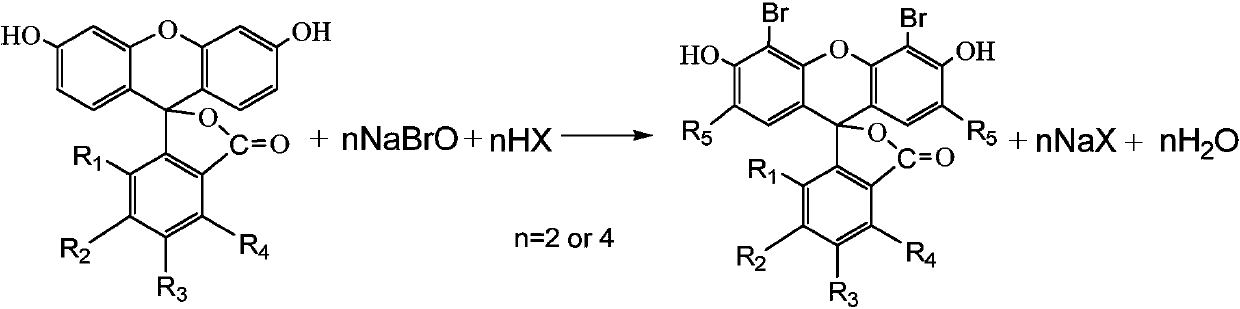
Preparation method of bromofluorescein type cosmetic dyestuff
A technology of fluorescein and cosmetics, applied in chemical instruments and methods, organic dyes, azo dyes, etc., can solve the problems of non-compliance with green chemistry and low utilization rate of bromine atoms, and achieve low price, easy acquisition, and high utilization rate Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 (preparation of 4', 5'-dibromofluorescein)
[0042] In a 250mL four-neck flask, add 30.5g of sodium hypochlorite solution (10%, 43mmol), place an ice-water bath outside, start stirring, add about 18g of water, 12.2g of 32% liquid caustic soda, and slowly drop Add 7.0 g of bromine (99%, 43 mmol), and control the reaction temperature below 15°C. Measure the content (available bromine is about 20%) and set it aside.
[0043] Add 100 g of 95% ethanol into a 500 mL four-neck flask, start stirring, add 14.3 g of fluorescein (97.5%, 42 mmol), heat up to 40-50° C., and dissolve. Add 13.6g of phosphoric acid (85%, 118mmol), cool down to 20-25°C, slowly add 67g of the above-mentioned sodium hypobromite solution (84mmol) dropwise within 1.5 hours, during which the reaction temperature is controlled at 20-25°C. Keep warm and stir for another 1 hour.
[0044] The temperature of the reaction solution was raised to 60° C., and about 80 g of ethanol was evaporated under va...
Embodiment 2
[0046] Example 2 (2', 4', 5', 7'-tetrabromofluorescein preparation)
[0047]Add in a 250mL four-neck flask, add 61.0g of sodium hypochlorite solution (10%, 86mmol), place an external ice-water bath, start stirring, add about 36g of water, 24.3g of 32% liquid caustic soda, after the temperature of the material reaches 0-5°C, slowly 13.9 g bromine (99%, 86 mmol) was added dropwise, and the reaction temperature was controlled below 15°C. Measure the content (available bromine is about 20%) and set it aside.
[0048] Add 250g of acetic acid into a 500mL four-neck flask, start stirring, add 13.6g of fluorescein (97.5%, 40mmol), heat up to 50-60°C, and stir rapidly (300-400rpm) for 30min. The temperature was lowered to 25-30°C, and 134.0 g of the above-mentioned sodium hypobromite solution (20%, 168 mmol) was slowly added dropwise within 3-4 hours, during which the reaction temperature was controlled at 25-30°C. Insulated and stirred for another 2 hours, a sample was taken to dete...
Embodiment 3
[0050] Example 3 (2', 4', 5', 7'-tetrabromofluorescein preparation)
[0051] Replace "13.6g fluorescein (97.5%, 40mmol)" with "12.9g fluorescein (97.5%, 38mmol)", and the rest are the same as in Example 2 to obtain 24.0g light pink target product (HPLC purity 97.6%, content 97.5%, 36mmol), yield 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com