A method, composition and application for preparing pluripotent stem cell-like cells
A technology of pluripotent stem cells and compositions, applied in the field of stem cell repair, can solve the problems of stress, difficult pluripotent stem cells, and high risk of cell canceration, and achieve the effects of reducing costs, simple operation, and low risk of canceration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
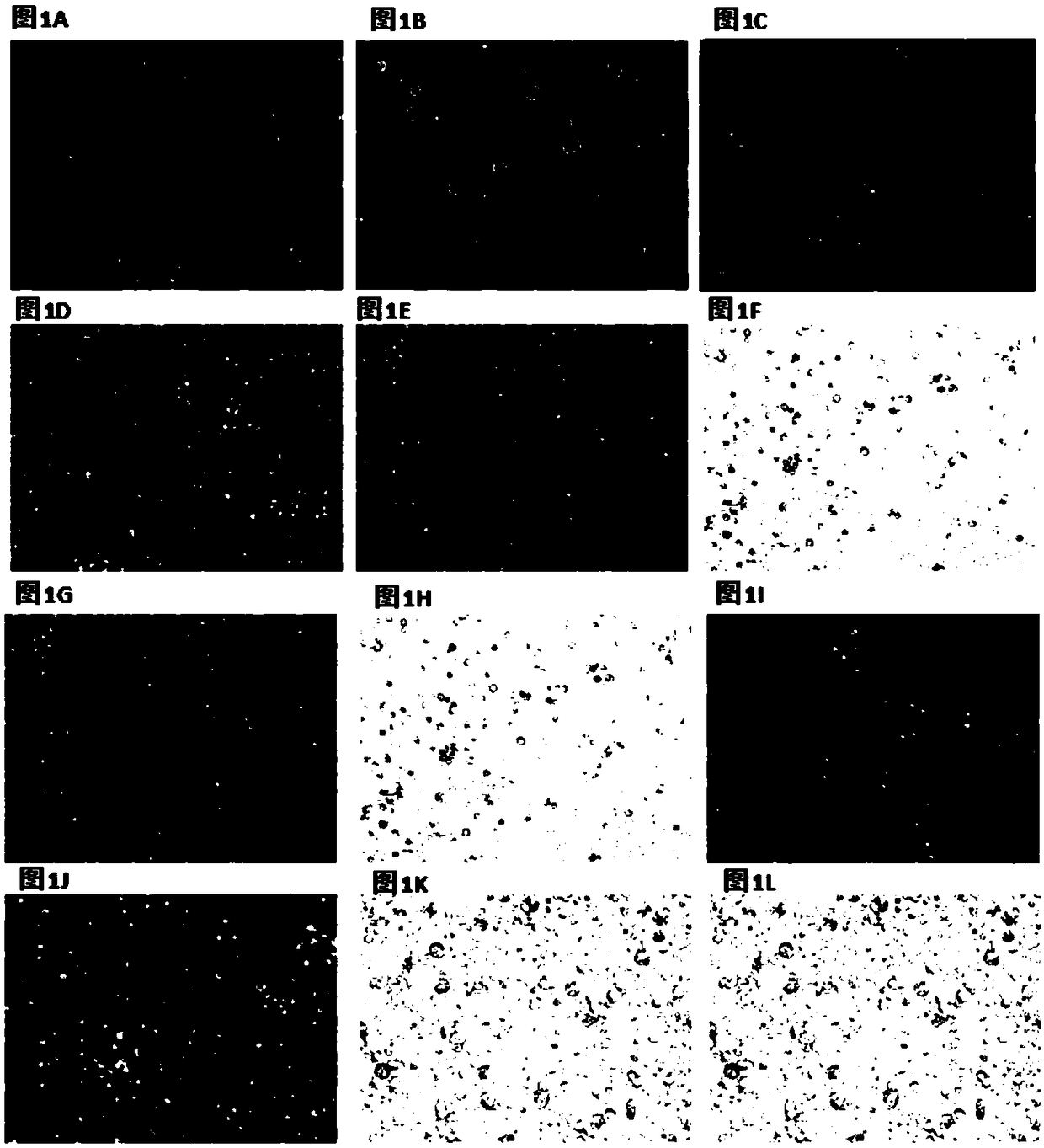
Image
Examples
Embodiment 1
[0108] Prepare peripheral blood-derived monocytes as follows:
[0109] 1) Collect peripheral blood samples. Take 4 sterile 50ml centrifuge tubes, carefully pour the samples into the centrifuge tubes, and put 50ml blood samples in each tube. 900g, centrifuge for 10min, slowly rise and fall slowly.
[0110] 2) Take the upper layer of yellow plasma into a new 50ml centrifuge tube (up to 3 / 4 of the serum layer to avoid loss of cell yield), and inactivate at 56°C for 15 minutes. Centrifuge at 3500rpm for 10min.
[0111] 3) Transfer the upper layer of plasma to a new 50ml centrifuge tube and store in a 4°C refrigerator for use.
[0112] 4) Resuspend the remaining blood sample to the original volume with PBS, prepare several sterile 50ml centrifuge tubes, add 20ml of human mononuclear cell separation solution to each tube, and do not splash the lymphocyte separation solution on the centrifuge tube wall (according to monocyte Separation solution: blood = 1:1, for example, 80ml who...
Embodiment 2
[0121] Drugs that induce dedifferentiation of monocytes to form pluripotent stem cell-like cells are screened in the following manner:
[0122] 1) Day 0: resuspend the monocytes prepared in Example 1, and make the density of the monocytes 1×10 6 / ml, inoculated into 24-well plates, at 37°C, 5% CO 2 Adhesive culture for 3 hours under the conditions; after that, the suspended cells were discarded, 0.5ml RPMI1640 medium was added to each well, and autologous serum was supplemented by 10%, and the cells were kept at 37°C and 5% CO 2 Continue to cultivate under the conditions of the medium, add M-CSF and autologous serum in the medium, and the final concentration of the M-CSF in the medium is 30ng / ml;
[0123] 2) Day 1, Day 2 and Day 3: Observe the cell morphology under the microscope every day.
[0124] Day3: Aspirate and discard 1 / 2 volume of medium in each well, and supplement 1 / 2 volume of fresh medium, which contains M-CSF (without adding the drug to be screened, as a contro...
Embodiment 3
[0134] Detect the ability of different types of Ophiopogon japonicus flavonoids to induce monocytes to form stem cell-like cells:
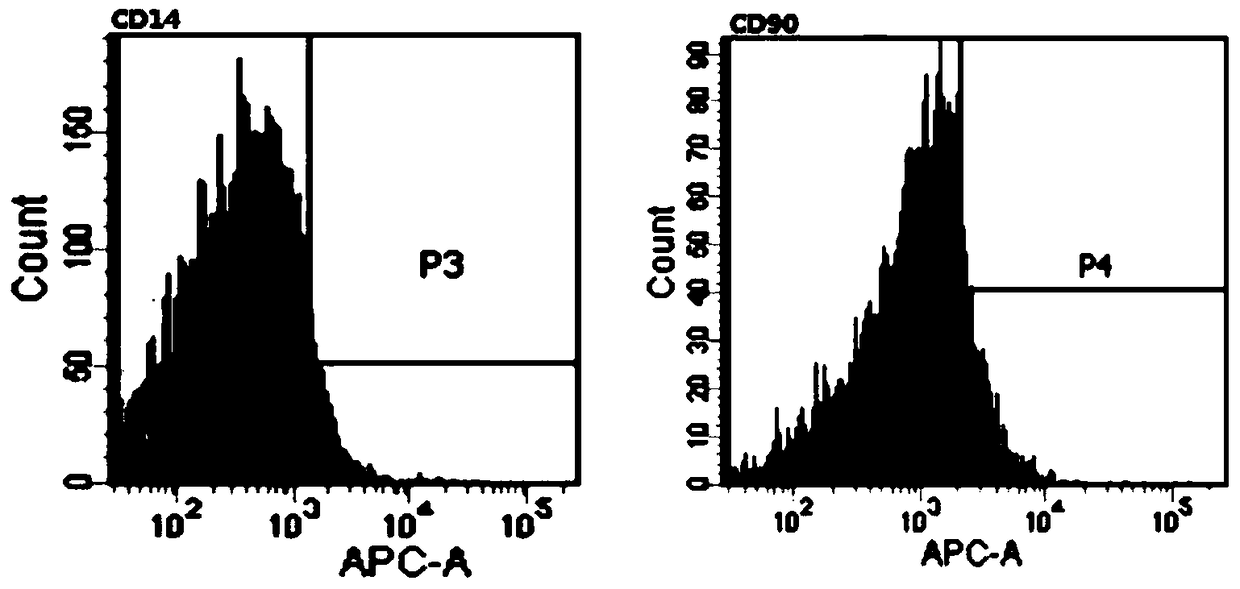
[0135] Refer to Example 2 for the specific method, the only difference is that the drugs to be tested are 6-formyl isotropis flavanone A, methyl radix flavanone B, methyl philopogon flavanone A, 6-formyl Isoflavone A and 6-formyl isoflavone B, see Table 2 for specific test results. According to the data shown in Table 2, it can be seen that the above five radix flavonoids can inhibit the expression of CD14 gene and up-regulate the expression of CD90 gene, and have the ability to induce monocytes to form stem cell-like cells. Among them, 6-formyl isoflavone A has the strongest inductive ability.
[0136] Table 2 The ability of different Ophiopogon japonicus flavonoids to induce monocytes to form stem cell-like cells
[0137]
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com