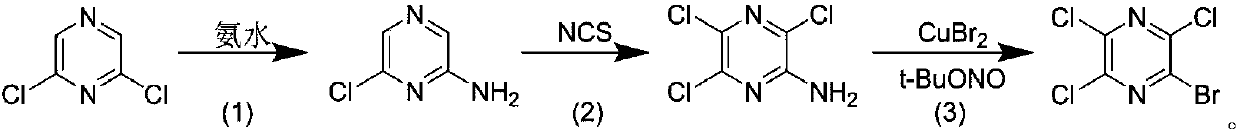
Preparation technology of 2-bromo-3,5,6-trichloropyrazine
A technology for the preparation of trichloropyrazine, which is applied in the field of preparation of 2-bromo-3,5,6-trichloropyrazine, can solve the problems of difficult product purification, low selectivity, and many by-products, and achieve easy Separation, high selectivity, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] A. Preparation of 2-amino-6-chloropyrazine
[0012] Add 2,6-dichloropyrazine (10.0 g, 67.1 mmol) and ammonia water (100 mL) into a hydrothermal reaction kettle, and react at 100° C. for 5 h. After the reaction was completed, it was cooled to room temperature, filtered with suction, and the filter cake was slurried with n-hexane (16 mL) to obtain 8.2 g of 2-amino-6-chloropyrazine as a white solid with a yield of 95.0%.
[0013] 1 H NMR (400 MHz, DMSO) δ: 7.70 (1H, d), 6.9 (2H, br s), 7.80 (1H, d). 13 C NMR (100 MHz, DMSO) δ 155.32, 141.92, 133.15, 131.15.
[0014] B. Preparation of 2-amino-3,5,6-trichloropyrazine
[0015] 2-Amino-6-chloropyrazine (2.0 g, 15.4 mmol) was dissolved in THF (50 mL), N-chlorosuccinimide (6.2 g, 46.3 mmol) was added, and the temperature was raised to reflux for 2 h. After the reaction was completed, cool to room temperature, pour the reaction solution into water, extract with ethyl acetate (40mL×3), collect the organic phase, dry over anhyd...
Embodiment 2
[0021] A. Preparation of 2-amino-6-chloropyrazine
[0022] 2,6-Dichloropyrazine (10.0 g, 67.1 mmol) and ammonia water (100 mL) were respectively added into a hydrothermal reaction kettle, and reacted at 60° C. for 18 h. After the reaction was completed, it was cooled to room temperature, filtered with suction, and the filter cake was slurried with n-hexane (16 mL) to obtain 6.3 g of 2-amino-6-chloropyrazine as a white solid with a yield of 72.5%.
[0023] 1 H NMR (400 MHz, DMSO) δ: 7.70 (1H, d), 6.9 (2H, br s), 7.80 (1H, d). 13 C NMR (100 MHz, DMSO) δ 155.32, 141.92, 133.15, 131.15.
[0024] B. Preparation of 2-amino-3,5,6-trichloropyrazine
[0025] 2-Amino-6-chloropyrazine (2.0 g, 15.4 mmol) was dissolved in THF (50 mL), N-chlorosuccinimide (4.1 g, 30.9 mmol) was added, and the temperature was raised to reflux for 3 h. After the reaction was completed, cool to room temperature, pour the reaction solution into water, extract with ethyl acetate (40mL×3), collect the organic...
Embodiment 3
[0030] A. Preparation of 2-amino-6-chloropyrazine
[0031] Add 2,6-dichloropyrazine (10.0 g, 67.1 mmol) and ammonia water (100 mL) into a hydrothermal reaction kettle, and react at 130° C. for 4 h. After the reaction was completed, it was cooled to room temperature, filtered with suction, and the filter cake was slurried with n-hexane (16 mL) to obtain 7.8 g of 2-amino-6-chloropyrazine as a white solid with a yield of 89.6%.
[0032] 1 H NMR (400 MHz, DMSO) δ: 7.70 (1H, d), 6.9 (2H, br s), 7.80 (1H, d). 13 C NMR (100 MHz, DMSO) δ 155.32, 141.92, 133.15, 131.15.
[0033] B. Preparation of 2-amino-3,5,6-trichloropyrazine
[0034] 2-Amino-6-chloropyrazine (2.0 g, 15.4 mmol) was dissolved in THF (50 mL), N-chlorosuccinimide (8.3 g, 61.8 mmol) was added, and the temperature was raised to reflux for 1 h. After the reaction was completed, cool to room temperature, pour the reaction solution into water, extract with ethyl acetate (40mL×3), collect the organic phase, dry over anhyd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com