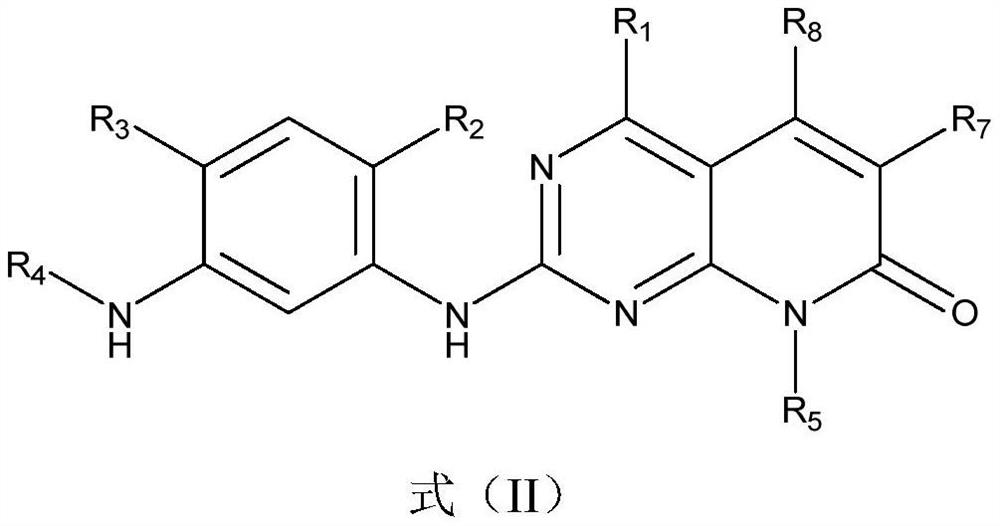
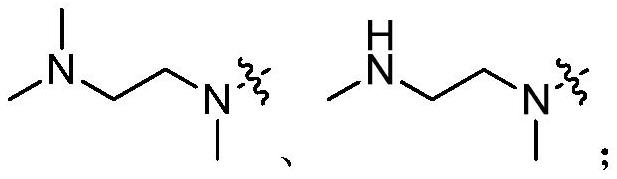
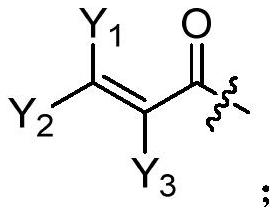
A kind of compound containing pyrimidine ring, egfr inhibitor and application thereof
A technology containing pyrimidine rings and compounds, applied in the field of medicine, can solve the problems of poor curative effect of drug-resistant patients, serious side effects of skin rashes of patients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0156] The compound containing pyrimidine ring in this embodiment has a structural formula as shown in formula (P-1):
[0157]
[0158]
[0159] The synthetic process of this compound is as follows:
[0160]
[0161] The synthetic method of this compound is: get the single mouth bottle of 100ml, add the raw material amine of 60mg (compound SM 2 -1), add 1.2eq of p-toluenesulfonic acid monohydrate crystals, add 1.2eq raw material pyrimidine compound (SM 1 -1) and 15ml of 2-pentanol, heated to 110°C overnight under stirring; the next day, distilled off the 2-pentanol, added 50ml of saturated aqueous sodium carbonate and 50ml of dichloromethane, dichloromethane extracted two Once, the organic phases were combined, dried, concentrated, and passed through the column to obtain 15 mg of the product. The analytical data of this compound are as follows: 1 H NMR (CDCl 3 )δ10.20(br,1H),8.75(m,2H),7.53(s,1H),7.10(m,3H),6.72(s,1H),6.36(m,3H),5.74(m,1H ),3.82(s,3H),2.84(br,2H)...
Embodiment 2
[0163] The compound containing pyrimidine ring of the present embodiment has a structural formula as shown in formula (P-2'):
[0164]
[0165] The synthetic process of this compound is as follows:
[0166]
[0167]Wherein, the synthesis method of compound (P-2) is the same as that of Example 1. The preparation method of compound P-2' (hydrochloride) in this example is: add 200 mg of compound P-2 into a 50 ml one-mouth bottle, add 10 ml of acetone and 1 ml of water, and slowly add it under stirring after the addition 140mg of hydrochloric acid with a mass concentration of 10%, reacted at room temperature for 3 hours after adding, evaporated the reaction solution to dryness, added 6ml of acetonitrile and raised the temperature to 70°C and stirred for 30 minutes, cooled slowly to precipitate the solid, filtered the solid, and used acetonitrile After washing and drying, 160 mg of white solid was obtained, which was the hydrochloride of compound P-2.
[0168] The analytica...
Embodiment 3
[0170] The compound containing pyrimidine ring of the present embodiment has a structural formula as shown in formula (P-3):
[0171]
[0172] The synthetic process of this compound is as follows:
[0173]
[0174] The synthetic method of this compound is with embodiment 1. The analytical data of this compound are as follows: 1 H NMR (CDCl 3 )δ10.10(br,1H),8.73(m,2H),7.56(s,1H),7.23(m,2H),7.10(m,2H),6.68(s,1H),6.43(m,3H ),5.74(m,1H),3.79(s,3H),2.85(m,2H),2.64(s,3H),2.44(s,3H),2.30(br,8H); MS m / z(ESI ):546.2[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com