Application of human TRIP13 gene and related medicine thereof
A gene and drug technology, applied in the use of human TRIP13 gene and related drug fields, can solve the problem of less TRIP13 gene and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Example 1 Preparation of RNAi lentivirus against human TRIP13 gene
[0069] 1. Screening for effective siRNA targets against the human TRIP13 gene
[0070] Retrieve TRIP13 (NM_004237) gene information from Genbank; design effective siRNA targets for TRIP13 gene. Table 1 lists the effective siRNA target sequences for the TRIP13 gene.
[0071] Table 1 is targeted at the siRNA target sequence of human TRIP13 gene
[0072] SEQ ID NO
Target Seq
1
gctactcaacagacatatat
[0073] 2. Preparation of lentiviral vector
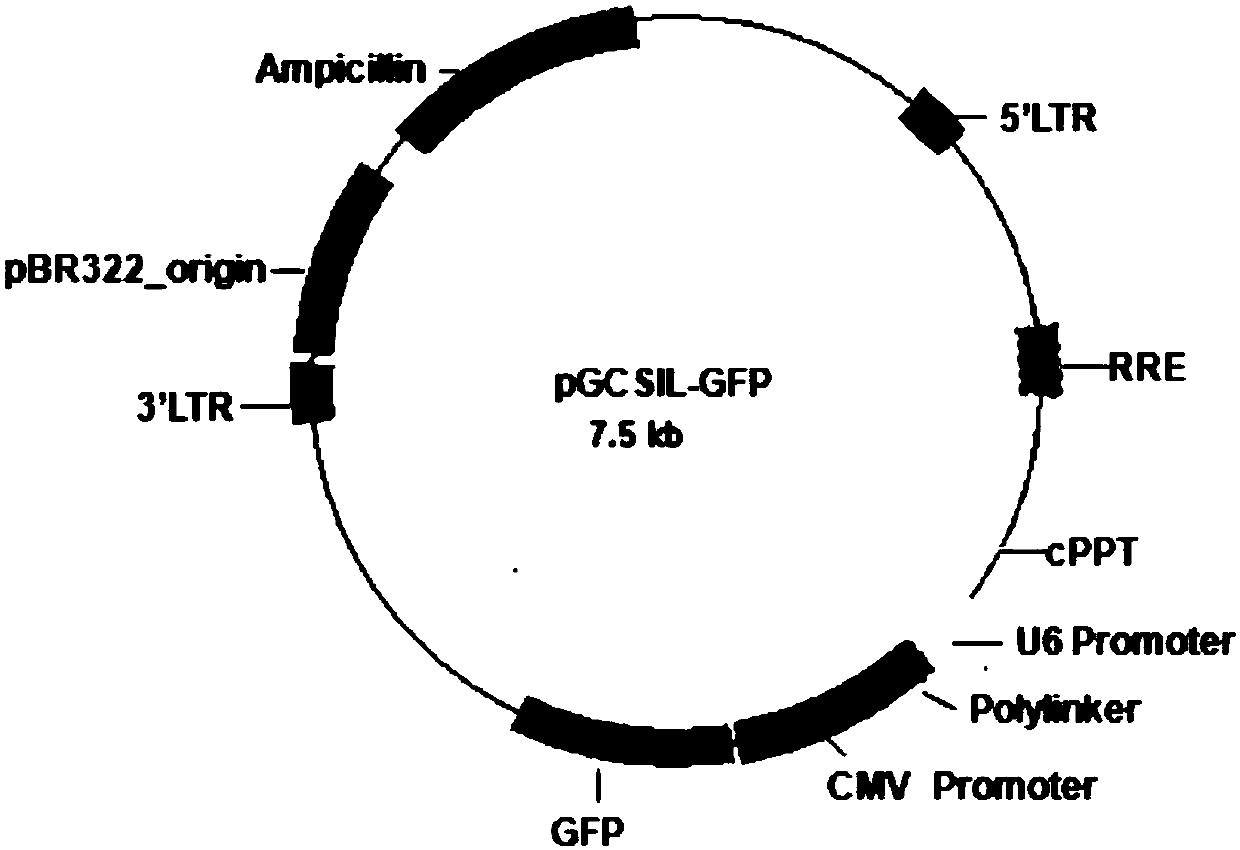
[0074] Aim at the siRNA target (taking SEQ ID NO: 1 as an example) to synthesize a double-stranded DNA Oligo sequence (Table 2) with Age I and EcoR I restriction endonucleases at both ends; use Age I and EcoR I restriction enzyme Act on the pGCSIL-GFP carrier (provided by Shanghai Jikai Gene Chemical Technology Co., Ltd., figure 1 ), to linearize it, and identify the digested fragments by agarose gel electrophoresis.
[0075] Table ...
Embodiment 2
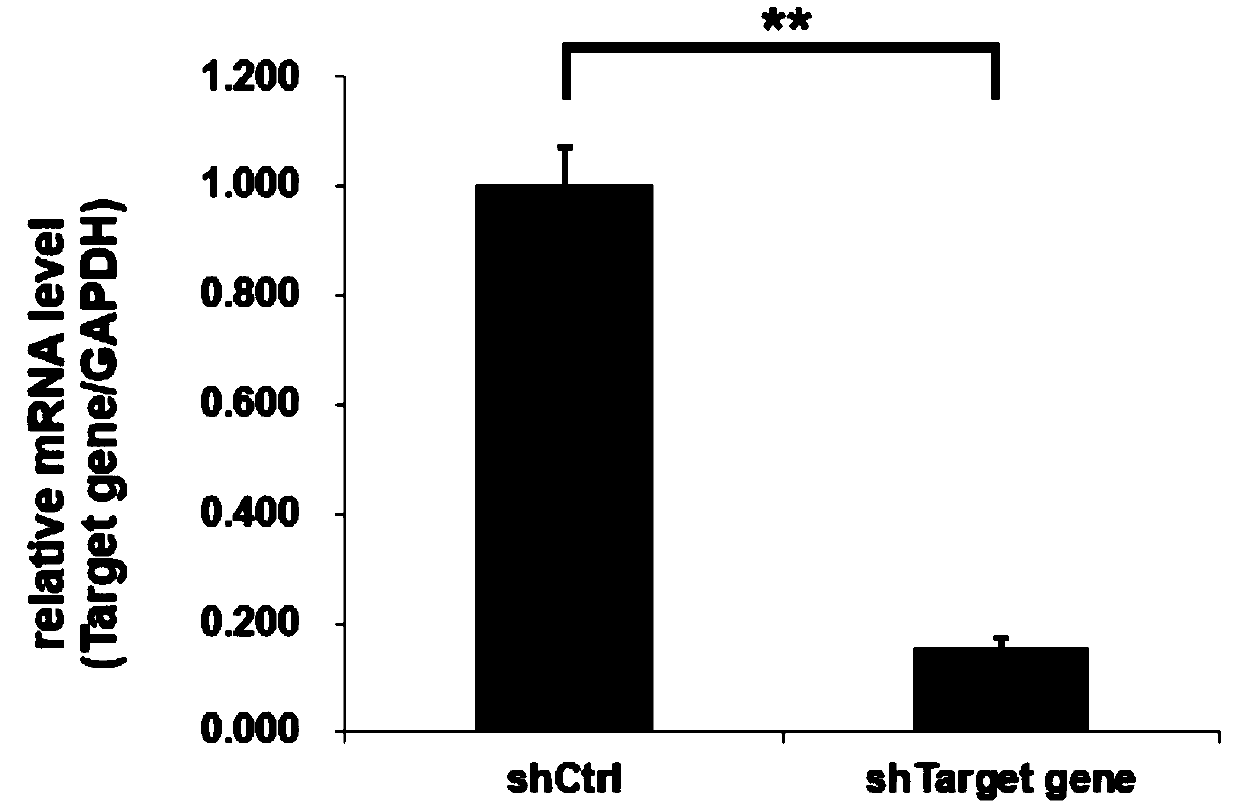
[0096] Example 2 Real-time fluorescent quantitative RT-PCR method to detect the silencing efficiency of TRIP13 gene
[0097] Human breast cancer MCF-7 cells in the logarithmic growth phase were digested with trypsin to make a cell suspension (the number of cells was about 5×10 4 / ml) were inoculated in a 6-well plate and cultured until the cell confluency reached about 30%. According to the multiplicity of infection (MOI, MCF-7:20) value, an appropriate amount of the virus prepared in Example 1 was added, the culture medium was replaced after 24 hours of cultivation, and the cells were collected after the infection time reached 5 days. Total RNA was extracted according to the instruction manual of Trizol from Invitrogen. According to the M-MLV instruction manual of Promega Company, RNA was reverse-transcribed to obtain cDNA (see Table 7 for the reverse transcription reaction system, react at 42° C. for 1 h, and then bathe in a water bath at 70° C. for 10 min to inactivate rev...
Embodiment 3
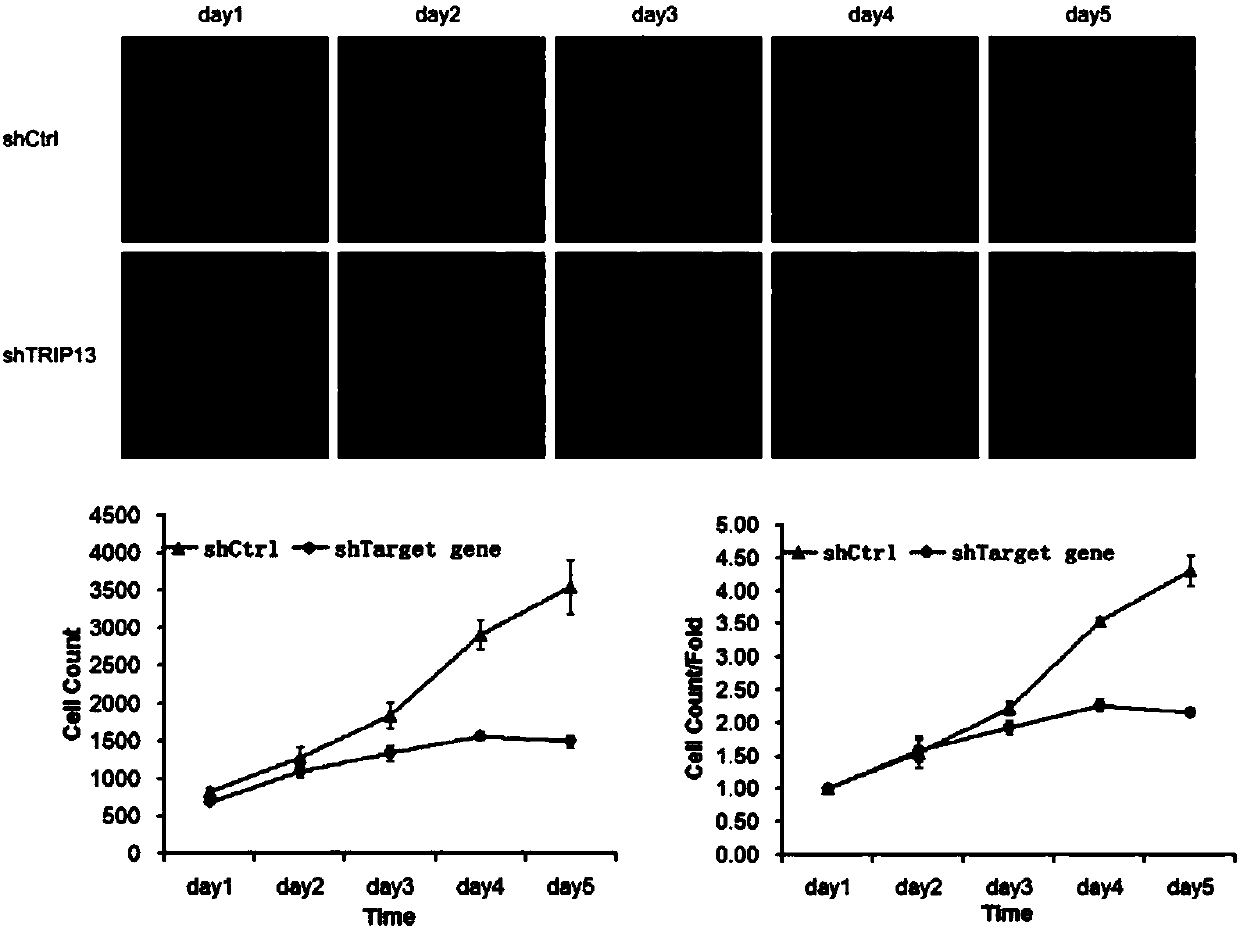
[0105] Example 3 Detection of proliferation ability of tumor cells infected with TRIP13-siRNA lentivirus
[0106] Human breast cancer MCF-7 cells in the logarithmic growth phase were digested with trypsin to make a cell suspension (the number of cells was about 5×10 4 / ml) were inoculated in a 6-well plate and cultured until the cell confluency reached about 30%. According to the multiplicity of infection (MOI, MCF-7:20), an appropriate amount of virus was added, and the culture medium was replaced after 24 hours of culture. After the infection time reached 5 days, the cells in each experimental group in the logarithmic growth phase were collected. The complete medium was resuspended into a cell suspension (2×10 4 / ml), inoculate a 96-well plate at a cell density of about 2000 / well. 5 replicate wells in each group, 100 μl per well. After laying the board, place at 37°C, 5% CO 2 Incubator cultivation. From the second day after plating, the plate was detected and read once ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com