Sodium potassium hydrogen citrate solution for aerosol inhalation
A technology of potassium hydrogen citrate and sodium aerosol inhalation, which is applied in the field of pharmacy and can solve the problems of large clinical adverse reactions and poor compliance of children
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
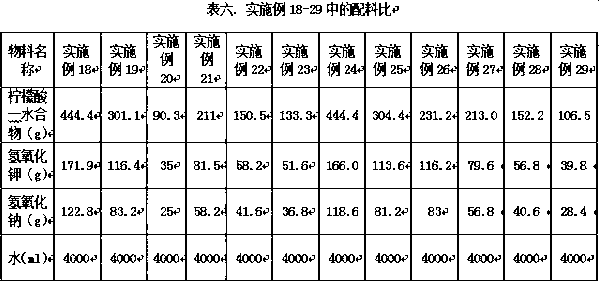
Embodiment 1-9
[0005] According to the ingredient ratio shown in Table 1, each material is mixed according to the corresponding ingredient ratio and mixed with water to the corresponding volume. During the preparation process, the acid is first and the alkali is added. The solid material is slowly added to the water and stirred while adding to prevent the temperature from rising. High, then, respectively sub-package into each preparation unit is 2ml solution preparation for nebulization inhalation, filled with nitrogen, sealed, closed light preservation.
[0006]
Embodiment 10
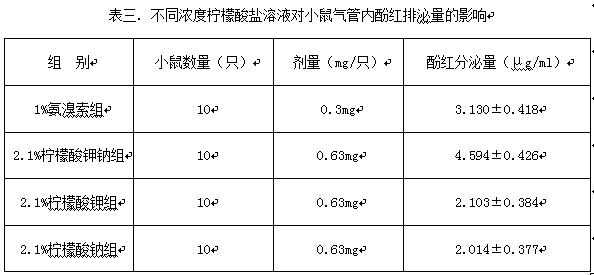
[0007] Embodiment 10 pharmacodynamic test
[0008] According to the ingredient ratio shown in Table 2, the pharmacodynamics experimental samples and control samples were prepared.
[0009]
[0010] According to the ingredient ratio shown in Table 2, add each material according to the corresponding ingredient ratio and mix with water for injection to a corresponding volume of 1000 ml. During the preparation process, first acid and then alkali, slowly add the solid material into the water, and stir while adding , to prevent the temperature from rising, and then, respectively aliquoted, nitrogen, sealed, and stored in a closed light, and the positive control sample was selected to have a concentration of 1.0% ambroxol solution. Take the weight at 20 + 1g of Kunming mice were starved overnight the day before the experiment and only provided drinking water. They were randomly divided into four groups, with 10 mice in each group. , inject 30 microliters of liquid medicine in ad...
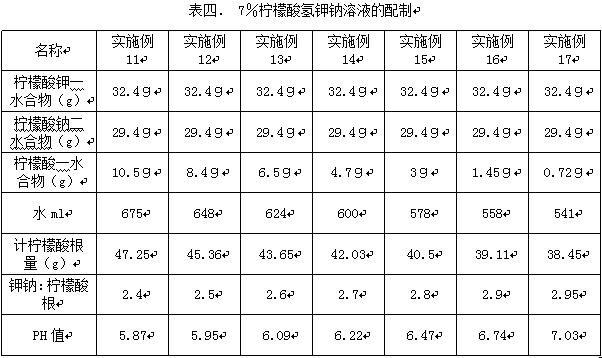
Embodiment 11-17
[0013] The influence of embodiment 11-17 potassium hydrogen citrate sodium hydrogen citrate on cilia movement
[0014] According to the ingredient ratio shown in Table 4, each material is mixed according to the corresponding ingredient ratio. The preparation process is first acid and then alkali. Slowly add the solid material into the water and stir while adding to prevent the temperature from rising. Pack each preparation unit into a solution preparation for atomization and inhalation of 2ml, fill with nitrogen, seal, and store in darkened light, and prepare a solution with the same citrate concentration of 7% and different potassium and sodium:citrate ratios.
[0015]
[0016] 24 domestic pigeons weighing 300±20 g were selected and randomly divided into 4 groups. Each group is followed by normal saline control group, 2.5 ratio of potassium sodium hydrogen citrate (embodiment 12) group, 2.7 ratio of potassium hydrogen citrate (embodiment 14) group, 2.95 ratio of potassium ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com