A preparing method of a cassava polysaccharide-iron complex
A cassava polysaccharide and cassava starch technology, which is applied in the field of fine chemicals, can solve the problems of high toxicity, high by-product glucose, and production safety hazards, so as to improve the ability of comprehensive utilization, reduce the production of glucose, and eliminate production safety hazards. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
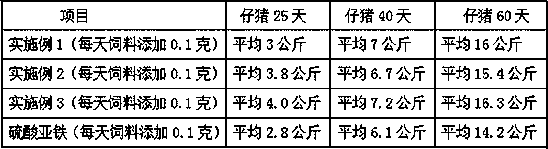
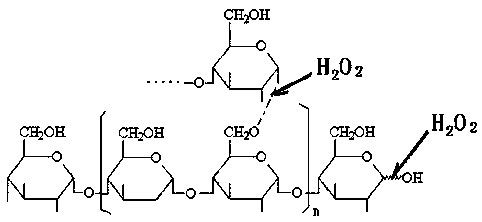
[0037] (1) Hydrogen peroxide oxidatively degrades tapioca starch to prepare suitable oligosaccharides: In a 200L enamel reaction kettle, start stirring, add 75.0 kg of water and 22.0 kg of tapioca starch, heat up to 60°C, and add 27.5% hydrogen peroxide 7.5 kg of solution, then heated up to 80°C, kept at a constant temperature, and carried out oxidation reaction for 60 minutes, when the reaction time was up, then added 29% sodium hydroxide seasoning solution with a pH value of 7.6, to obtain the suitable low-density compound with a weight average molecular weight of 4000-27000Da. polysaccharide solution.
[0038] (2) Complexation reaction: keep stirring, heat the prepared suitable oligosaccharide solution to 90°C, keep constant temperature, add 52 liters of 39.0% ferric chloride solution and 56 liters of 29.0% sodium hydroxide solution at the same time, Added within 3 hours. After adding the materials, adjust the pH value of the complexation reaction solution to 7.3 with 20% ...
Embodiment 2
[0043] ⑴ Hydrogen peroxide oxidatively degrades tapioca starch to prepare suitable oligosaccharides: In a 200L enamel reaction kettle, start stirring, add 80.0 kg of water and 22.0 kg of tapioca starch, heat up to 60°C, and add 27.5% hydrogen peroxide solution 8.5 kg, then raise the temperature to 88°C, keep the constant temperature, carry out the oxidation reaction for 45 minutes, when the reaction time is up, add 29% sodium hydroxide seasoning solution and the pH value is 7.2, that is, the suitable oligomer with a weight average molecular weight of 4000-27000Da sugar solution.
[0044] (2) Complexation reaction: keep stirring, heat the prepared suitable oligosaccharide solution to 95°C, keep the constant temperature, add 52 liters of 39.0% ferric chloride solution and 56 liters of 29.0% sodium hydroxide solution at the same time, in 2.5 hours Added. After adding the materials, adjust the pH value of the complexation reaction solution to 7.6 with 20% hydrochloric acid to obt...
Embodiment 3
[0049] ⑴ Hydrogen peroxide oxidatively degrades tapioca starch to prepare suitable oligosaccharides: In a 200L enamel reaction kettle, start stirring, add 85.0 kg of water and 22.0 kg of tapioca starch, heat up to 60°C, add 27.5% hydrogen peroxide solution 9.5 Then raise the temperature to 95°C, keep constant temperature, carry out the oxidation reaction for 30 minutes, when the reaction time is up, add 29% sodium hydroxide seasoning solution with a pH value of 8.0, and obtain suitable oligosaccharides with a weight average molecular weight of 4000-27000Da solution.
[0050] (2) Complexation reaction: keep stirring, heat the prepared suitable oligosaccharide solution to 85°C, keep a constant temperature, add 52 liters of 39.0% ferric chloride solution and 56 liters of 29.0% sodium hydroxide solution at the same time, in 2 Added within hours. After adding the materials, adjust the pH value of the complexation reaction solution to 8.0 with 20% hydrochloric acid to obtain the ca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com