A kind of synthetic method of biochemical preparation tmb
A synthesis method and preparation technology, applied in the field of biochemical preparation TMB synthesis, can solve the problems of lack of data support, no use value, no operability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
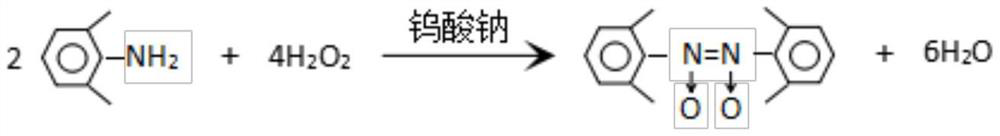
[0036] 1. Synthesis of 2,2′,6,6′-azotetramethylaniline-N,N-dioxide:
[0037]
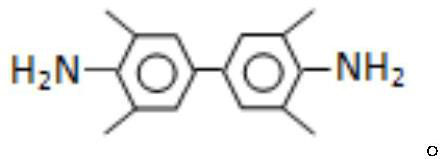
[0038] In a 2000ml reaction flask, add 290.4g 2,6-xylidine (light red, purity over 97%), 348g water, 59g sodium tungstate dihydrate and 615ml ether, stir evenly, cool down to 20°C, pour into the reaction flask Add 451ml of 30wt% hydrogen peroxide solution dropwise to the solution, add dropwise for 1h, control the temperature of the reaction bottle at 20-30°C during the dropwise addition, continue to insulate and stir the reaction for 8h after dropping, after the reaction is completed, cool down to 5°C, precipitate and filter , wash the filter cake 3 times with 96ml of cold diethyl ether, and dry it in the air to obtain 318.7g of 2,2′,6,6′-azotetramethylaniline-N,N-dioxide, reddish brown, mp134~135℃, purity 99.3%, yield 98%.
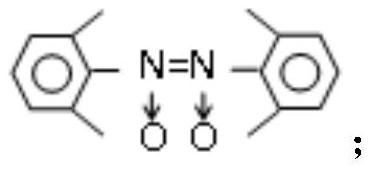
[0039] Its structural formula is as follows:
[0040]
[0041] Elemental analysis; C: 80.9; H: 7.3; N: 11.4%; -1 .
[0042] The ether in the filtrate can be reclaimed...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com