Novel thiazole derivative compound as well as preparation method, pharmaceutical composition and pharmaceutical application of novel thiazole derivative compound
A compound, thiazole technology, applied in the field of thiazole derivatives, can solve the problems of limited tumor cell inhibitory ability, limited influence of downstream signals, and drug resistance, and achieve high tumor cell proliferation inhibitory ability, weak normal cell toxicity, and increased The effect of binding ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
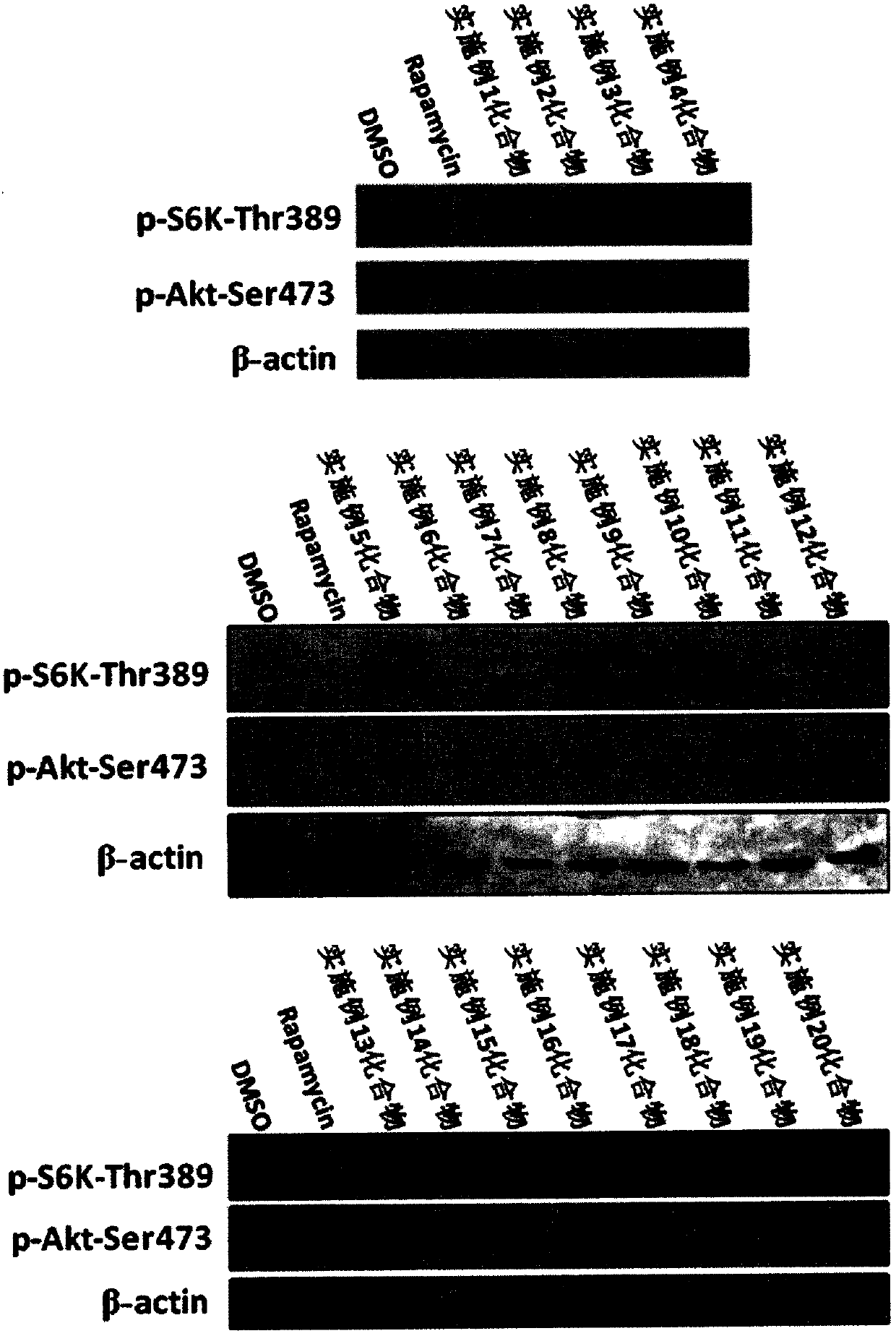
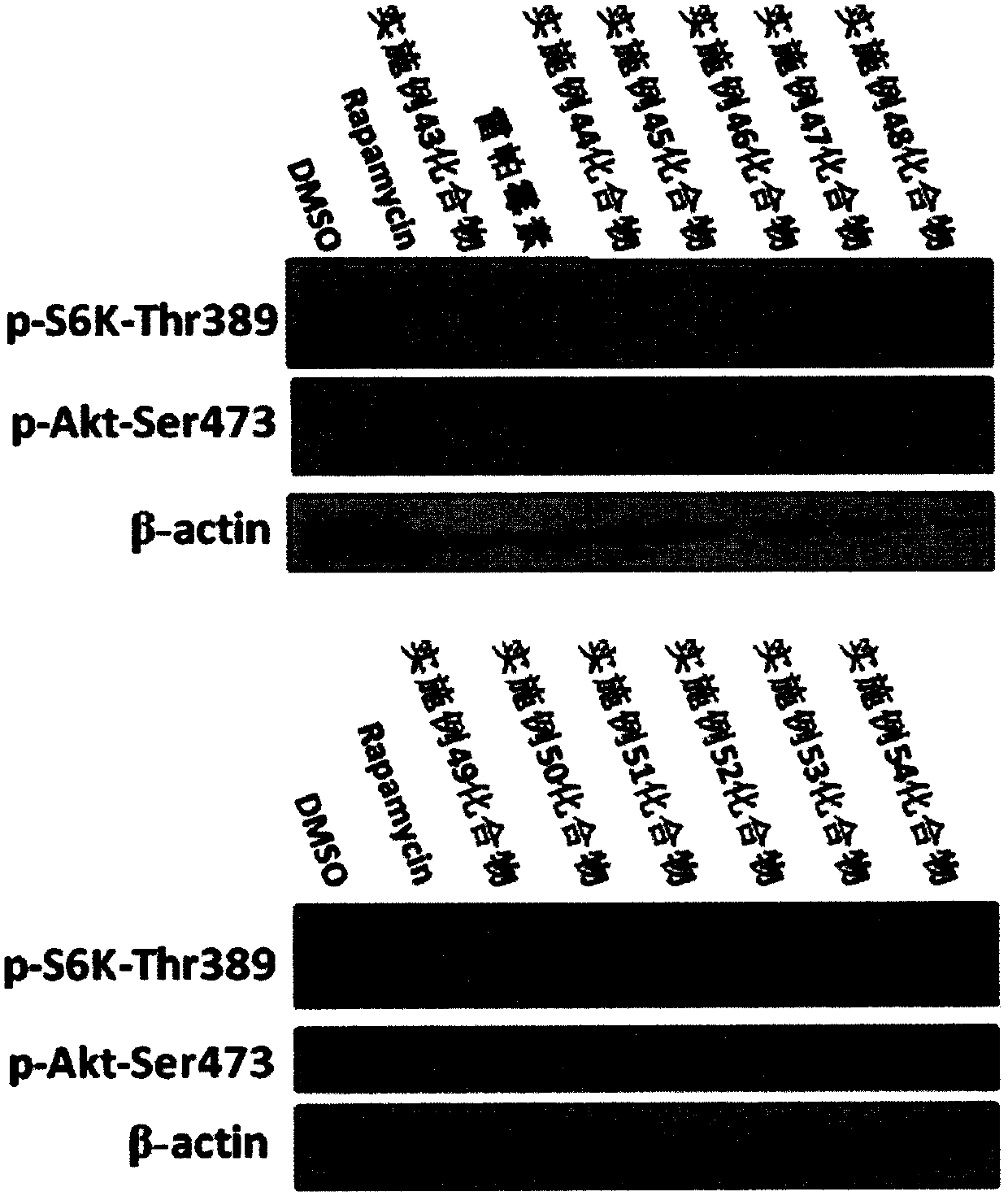
Image
Examples
Embodiment 1
[0131] Example 1: Preparation of 1-(4-(2,4-dimorpholinobenzothiazol-6-yl)phenyl)-3-ethylurea
[0132]
[0133] Step 1: Preparation of 2,4,6-tribromoisothiocyanate (Intermediate 1)
[0134] Add 2,4,6-tribromoaniline (20g, 60.6mmol) into dichloromethane (100ml) at room temperature, and add thiophosgene (20.9g, 181.8mmol) to dichloromethane once dissolved . DMAP (4-dimethylaminopyridine) (37.01 g, 303 mmol) was dissolved in dichloromethane (100 ml), and slowly added dropwise to the reaction solution within 1 h, and the reaction was stopped after stirring for 5 hours. Column chromatography gave 2,4,6-tribromoisothiocyanate (21.41 g, 57.6 mmol, yield 95%).
[0135] Step 2: Preparation of 2-morpholino-4,6-dibromo-benzothiazole (intermediate 2)
[0136] 2,4,6-Tribromoisothiocyanate (21.41g, 57.6mmol) was dissolved in dioxane (250ml) at room temperature, morpholine (5.27g, 60.48mmol) was added, and the reaction was stirred for 30min. After all the raw materials were reacted, ce...
Embodiment 2
[0140] Example 2: Preparation of 1-cyclopropyl-3-(4-(2,4-dimorpholinobenzothiazol-6-yl)phenyl)urea
[0141]
[0142] Carry out synthesis according to the synthesis method of the compound of Example 1, change the 6-position side chain into 4-isopropylurea-phenylboronic acid pinacol ester, obtain white powder 1-cyclopropyl-3-(4-(2,4 - pure dimorpholinobenzothiazol-6-yl)phenyl)urea (total yield: 27.2%). m.p.238-240℃; 1 H-NMR (400MHz, DMSO-d 6 δppm), 8.39(s, 1H), 7.64(s, 1H), 7.56(d, 2H, J=8.0Hz), 7.47(d, 2H, J=8.0Hz), 6.96(s, 1H), 6.41(s , 1H), 3.80(s, 4H), 3.75(s, 4H), 3.53(s, 4H), 3.38(s, 4H), 3.12(m, 2H), 1.99(s, 1H), 1.17(t, 1H, J=8.0Hz), 0.65(d, 2H, J=8.0Hz), 0.41(s, 2H); EI-MS: 480.1[M+1] + .
Embodiment 3
[0143] Example 3: Preparation of 1-benzyl-3-(4-(2,4-dimorpholinobenzothiazol-6-yl)phenyl)urea
[0144]
[0145]Carry out synthesis according to the synthesis method of the compound of Example 1, change the 6-position side chain into 4-benzyl urea-phenylboronic acid pinacol ester, obtain white powder 1-benzyl-3-(4-(2,4- Pure dimorpholinobenzothiazol-6-yl)phenyl)urea mTOR-20 (total yield: 24.5%). m.p.252-255℃; 1 H-NMR (400MHz, DMSO-d 6 δppm), 8.68(s, 1H), 7.64(s, 1H), 7.58(d, 2H, J=8.0Hz), 7.48(d, 2H, J=8.0Hz), 7.33(m, 4H), 7.25(m , 1H), 6.96(s, 1H), 6.66(t, 1H, J=4.0Hz), 4.32(s, 2H, J=4.0Hz), 3.80(s, 4H), 3.75(s, 4H), 3.54 (s, 8H); EI-MS: 530.2[M+1] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com