Standard product for detection of clinical drug-resistant genes in intestinal cancer and application thereof
A drug-resistant gene and standard product technology, which is applied in the determination/testing of microorganisms, DNA/RNA fragments, recombinant DNA technology, etc., can solve the problems of lack of diversity and the existence of test results, and achieve the effect of accurate guidance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0029] It should be noted that, in the case of no conflict, the embodiments in the present application and the features in the embodiments can be combined with each other. The present invention will be described in detail below in conjunction with examples.
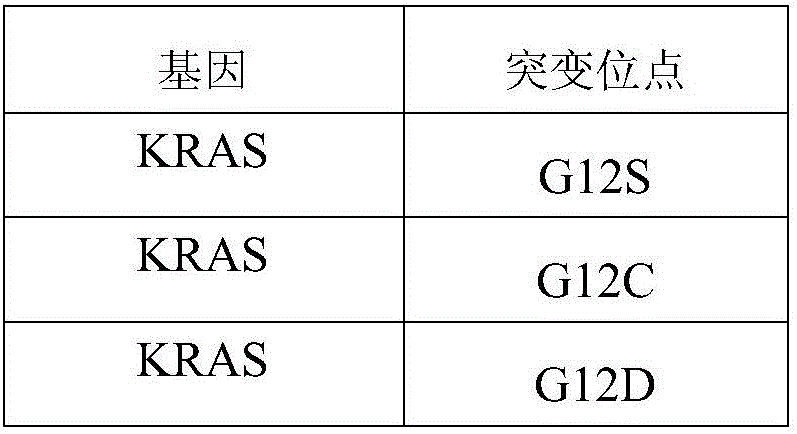
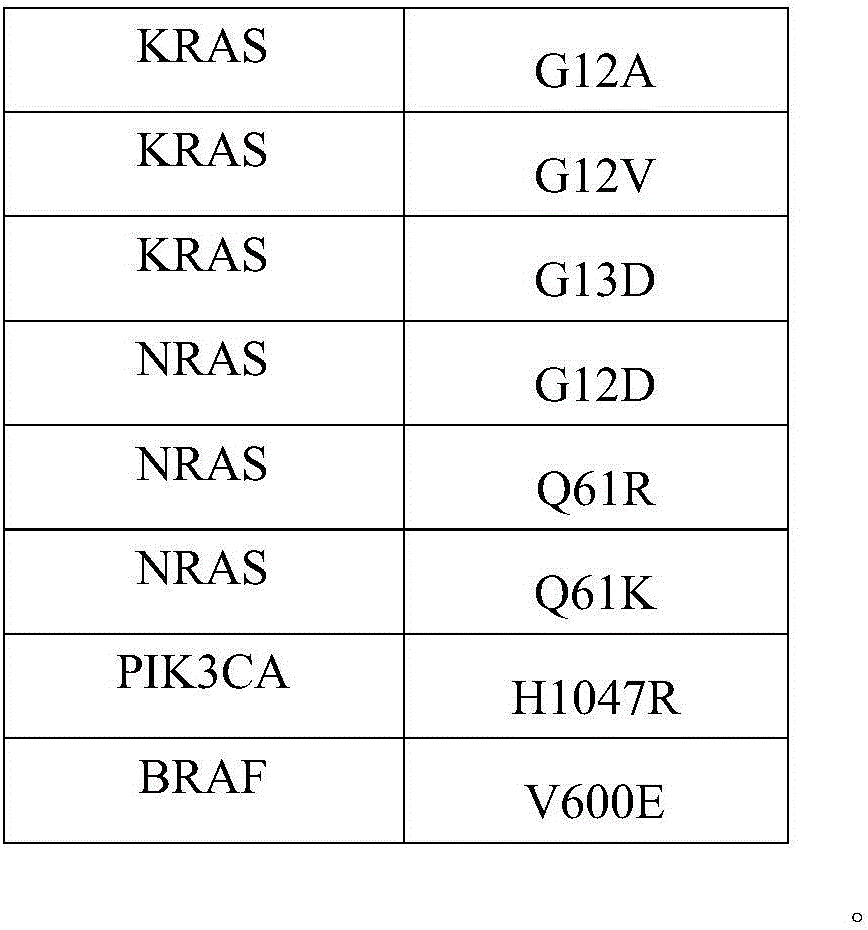
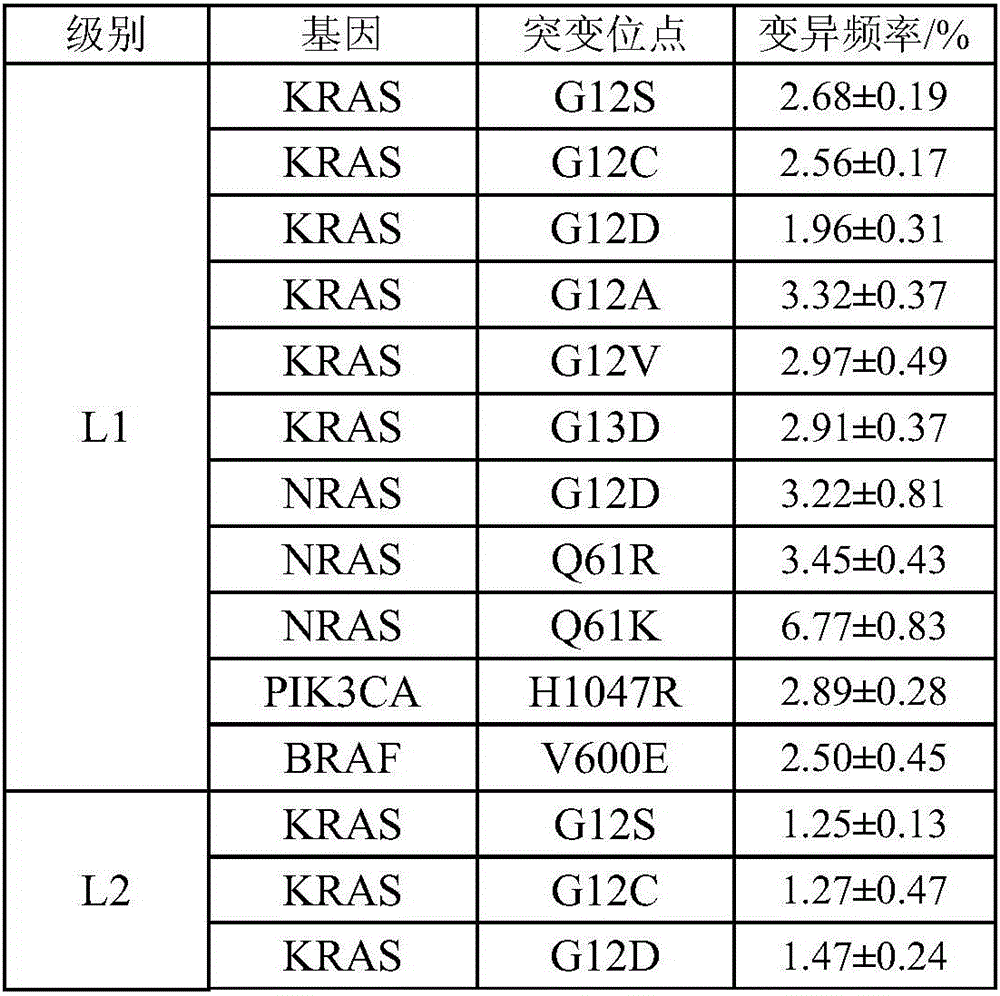
[0030] As mentioned in the background technology, the detection results of the detection kits for gene mutations related to cancer in the prior art are diverse and lack clinical guiding significance, and there is currently no effective solution. In a typical embodiment of the present application, a standard product for clinical drug resistance gene detection of colorectal cancer is provided, the standard product includes: a minimum detection limit reference product, the minimum detection limit reference product includes at least two different levels of variation The frequency DNA mixture, the DNA mixture of each level of variation frequency includes the mutation sites shown in Table 1 below:
[0031] Table 1:
[0032] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com