Method for preparing methyl glycollate and byproduct methyl methoxy acetate by molecular sieve catalyst
A technology for by-producing methyl methoxyacetate and methyl glycolate is applied in the directions of carbon monoxide or formate reaction preparation, chemical instruments and methods, preparation of organic compounds, etc., and can solve the problems of polluted environment, low product selectivity, Problems such as low conversion rate of raw materials, to achieve the effect of increasing the ratio of CO and aldehyde groups
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Embodiment 1: catalyst preparation example
[0071] H-Y catalyst
[0072] Exchange 100 grams of calcined Na-Y (Si / Al=6.5) molecular sieves with 0.5mol / L ammonium nitrate three times, each time for 2 hours, wash with deionized water, dry, calcinate at 550°C for 4 hours, and extrude 1# catalysts of 20-40 mesh were prepared respectively.
[0073] Supported M / Y catalyst
[0074] The supported 5% M / Y catalyst was prepared by isometric impregnation method. 4.32 g Cu(NO 3 ) 2 ·3H 2 O, 3.04 gAgNO 3 ·3H 2 O, 4.32gFe(NO 3 ) 3 , 5.21g Co(NO 3 ) 2 ·6H 2 O , 5.21 g Ni(NO 3 ) 2 ·6H 2 O and 4.58 g Ga(NO 3 ) 3 Dissolve in 18 mL deionized water to make the corresponding nitrate aqueous solution. Add 20g of 1# H-Y molecular sieve catalyst to the above-mentioned nitrate aqueous solution respectively, let it stand for 24 hours, then separate it, wash it with deionized water, dry the obtained sample in an oven at 120°C for 12 hours, and place the dried sample in a muffle f...
Embodiment 2
[0085] Embodiment 2: the reactivity performance of catalyst
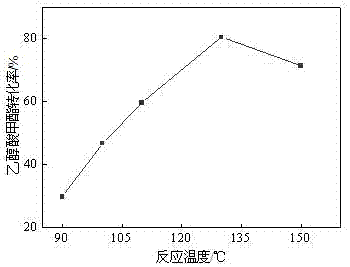
[0086] The catalyst powder obtained in Example 1 was tabletted, crushed, and sieved to obtain a 40-60 mesh sample for the synthesis of methyl glycolate and the determination of the reaction performance of the by-product methyl methoxyacetate. Weigh 20 kg of methylal (DMM), 3.9 kg of paraformaldehyde, 4 kg of water, and 300 g of various catalysts prepared in Example 1 and put them into the reaction kettle, and then feed 1.0 MPa of carbon monoxide gas. After emptying the gas in the reactor, repeat the above operation 2 times (replacing the air in the reactor). Introduce a certain amount of gas (6.0 MPa) again to test for leaks, and let it stand for 15 minutes. If the pressure indicator does not drop, it means that there is no air leakage in the device. Then empty the gas in the kettle and fill the kettle with 6.0 MPa CO gas again. , heated up, the stirring speed of the reactor was 500 rpm, the reaction pressure was 6...
Embodiment 3
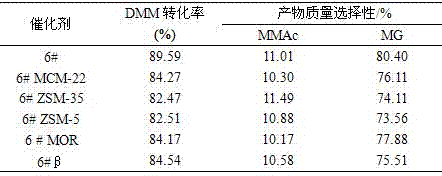
[0092] Respectively with hydrogen type Y molecular sieve, hydrogen type MCM-22 molecular sieve, hydrogen type ZSM-35 molecular sieve, hydrogen type ZSM-5 molecular sieve, hydrogen type mordenite, hydrogen type Beta molecular sieve as the metal modified catalyst carrier, with 6 in embodiment 1 The preparation method of #catalyst prepares metal-modified catalyst, respectively marked as 6#, 6# MCM-22, 6# ZSM-35, 6# ZSM-5, 6# MOR, 6#β catalyst, and gained catalyst powder is through tabletting The 40-60 mesh samples obtained by grinding, crushing and sieving were used for the synthesis of methyl glycolate and the determination of the reaction performance of the by-product methyl methoxyacetate. Weigh 20 kg of methylal (DMM), 3.9 kg of paraformaldehyde, 4 kg of water, and 300 g of the above-mentioned catalysts into the reaction kettle, and then feed 1.0 MPa of carbon monoxide gas, and empty the gas in the kettle if there is no leakage After that, repeat the above operation 2 times (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com