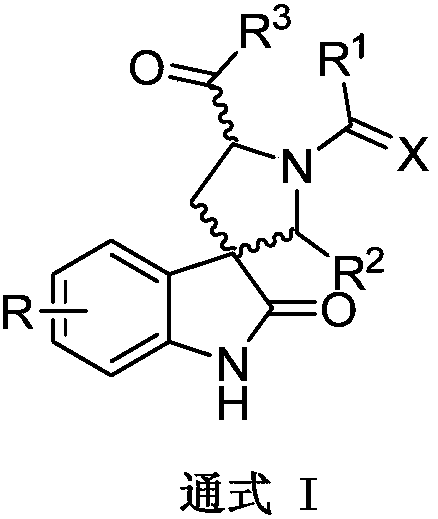
Spirooxoindole (thio)hydantoin derivative as well as preparation method and application thereof to aspects of plant virus prevention and control, sterilization and insect killing
A technology of indole hydantoin and urea derivatives is applied in the field of pesticides, which can solve the problems of no literature reports on anti-plant virus activity, bactericidal activity and insecticidal activity, and achieve excellent anti-plant virus activity and good anti-TMV in vivo activity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
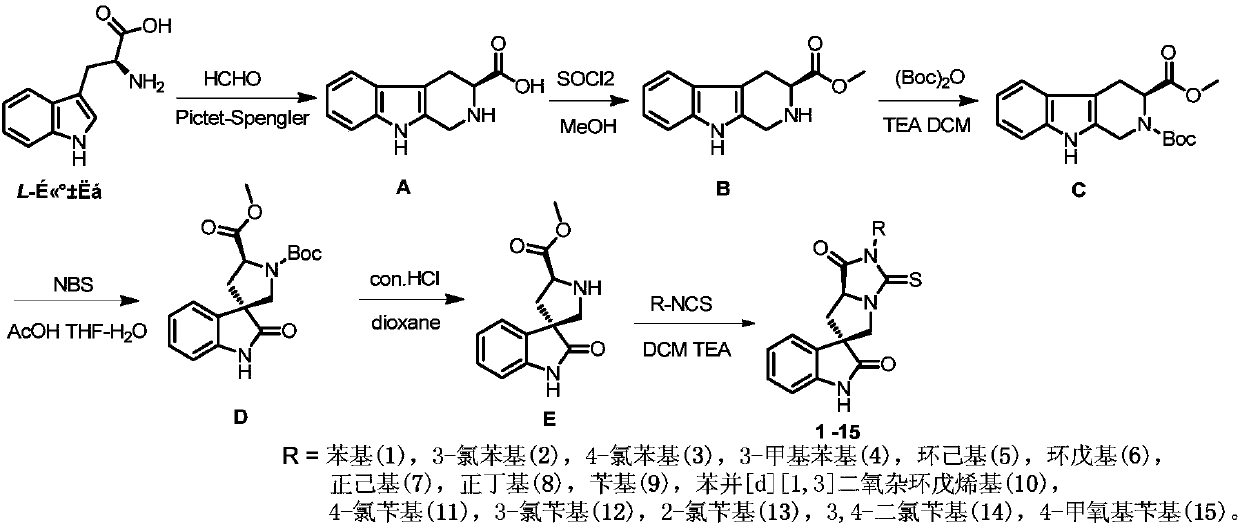
[0038]Embodiment 1: the synthesis (1-15) of spiroepoxide indole thiohydantoin derivative
[0039]
[0040] route one
[0041] Synthesis of (S)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylic acid (A)
[0042] Add L-tryptophan (30 g, 0.147 mol), NaOH (5.88 g, 0.147 mol) and 400 mL of water into a 1 L single-necked flask, and stir until clear. Then 18 mL of 30% formaldehyde solution was added, and heated to reflux for 3 h. After cooling, the pH was adjusted with 2mol / L hydrochloric acid solution. At this time, a precipitate was formed, which was filtered, washed and dried to obtain 24.58 g of a khaki solid, with a yield of 77.4%. 1 H NMR (400 MHz, DMSO-d 6 )δ 10.96(s, 1H), 7.44(d, J=7.5Hz, 1H), 7.33(d, J=7.3Hz, 1H), 7.07(t, J=7.2Hz, 1H), 6.99(t, J =7.6Hz, 1H), 4.31-4.20(m, 2H), 3.64(dd, J=9.6, 4.4Hz, 1H), 3.35(brs, 1H), 3.15(dd, J=15.8, 4.4Hz, 1H) , 2.83 (dd, J=15.7, 10.7Hz, 1H).
[0043] Synthesis of (S)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylic aci...
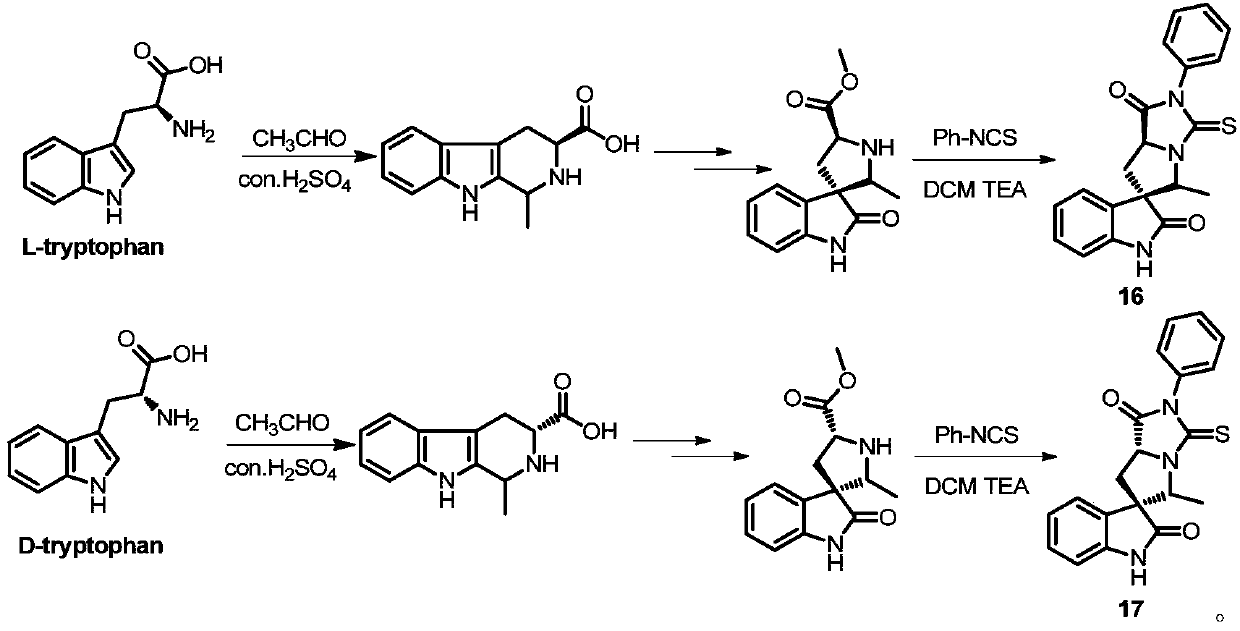
Embodiment 2
[0082] Example 2: Synthesis of spiro-epoxyindole thiohydantoin derivatives (16, 17)
[0083]
[0084] route two
[0085] Compounds 16 and 17 were synthesized according to compound 1.
[0086] (3S, 5'R, 7a'R)-5'-methyl-2'-phenyl-3'-thio-2',3',7',7a'-tetrahydrospiro[indoline- 3,6'-pyrrolo[1,2-c]imidazole]-1',2(5'H)-dione (16)
[0087] Yellow powder, yield 84.3%, melting point 135-137°C. 1 H NMR (400MHz, CDCl 3 )δ 9.61(s, 1H), 7.57-7.48(m, 5H), 7.30(s, 1H), 7.22-7.17(m, 1H), 7.10-7.05(m, 1H), 6.75(d, J=7.7 Hz, 1H), 4.96-4.89(m, 2H), 2.72(d, J=7.7Hz, 2H), 1.44(d, J=6.9Hz, 3H). 13 C NMR (100MHz, CDCl 3 for C 20 h 17 N 3 o 2 S(M+H) + 364.1114, found 364.1118.
[0088] (3R, 5'S, 7a'R)-5'-methyl-2'-phenyl-3'-thio-2',3',7',7a'-tetrahydrospiro[indoline- 3,6'-pyrrolo[1,2-c]imidazole]-1',2(5'H)-dione (17)
[0089]Yellow powder, yield 68.9%, melting point 106-109°C. 1 H NMR (400MHz, CDCl 3 ( m, 1H), 5.56-5.50 and 5.19-5.13 and 4.96-4.88, 4.53-4.46 (m, 2H), 3.46-3.28 (m...
Embodiment 3
[0090] Embodiment 3: the synthesis (18) of spiroepoxide indole thiohydantoin derivative
[0091]
[0092] Refer to compound 1 for the synthetic operation steps of route 3 compound 18.
[0093] (3S, 5′S)-1′-(tert-butylthiocarbamoyl)-2-oxospiro[indoline-3,3′-pyrrolidine]-5′-methyl carboxylate ( 18)
[0094] White powder, yield 45.7%, melting point 108-110°C. 1 H NMR (400MHz, CDCl 3 )δ 9.44(s, 1H), 7.26 (t, J=7.4Hz, 1H), 7.13(d, J=7.2Hz, 1H), 7.06(t, J=7.4Hz, 1H), 7.01(d, J =7.7Hz, 1H), 5.78(s, 1H), 5.32(t, J=8.2Hz, 1H), 4.00(d, J=9.8Hz, 1H), 3.82(s, 1H), 3.79(s, 3H ), 2.61-2.51(m, 1H), 2.47-2.36(m, 1H), 1.52(s, 9H). 13 C NMR (100MHz, CDCl 3 )δ 180.2, 177.7, 172.4, 140.1, 132.3, 129.0, 123.5, 122.7, 110.6, 62.4, 56.9, 54.4, 52.8, 52.7, 39.0, 29.0. HRMS (ESI) calcd for C 18 h 23 N 3 o 3 S(M+H) + 362.1533, found 362.1538.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com