Lung cancer clinical drug gene detection standard and its application
A technology of genetic testing and standard products, which is applied in the field of biomedicine, can solve the problem that the detection sensitivity cannot be standardized and evaluated, and achieve the effect of accurate guidance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] 1. Preparation of standard products
[0056] (1) Raw material
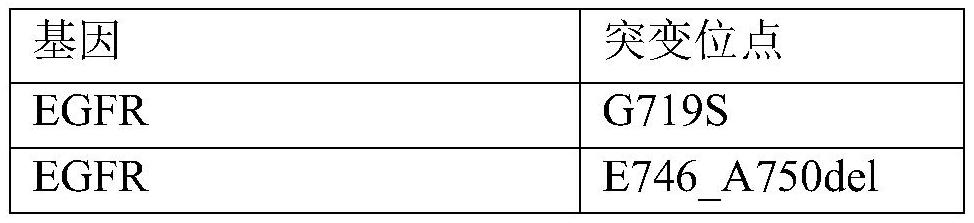
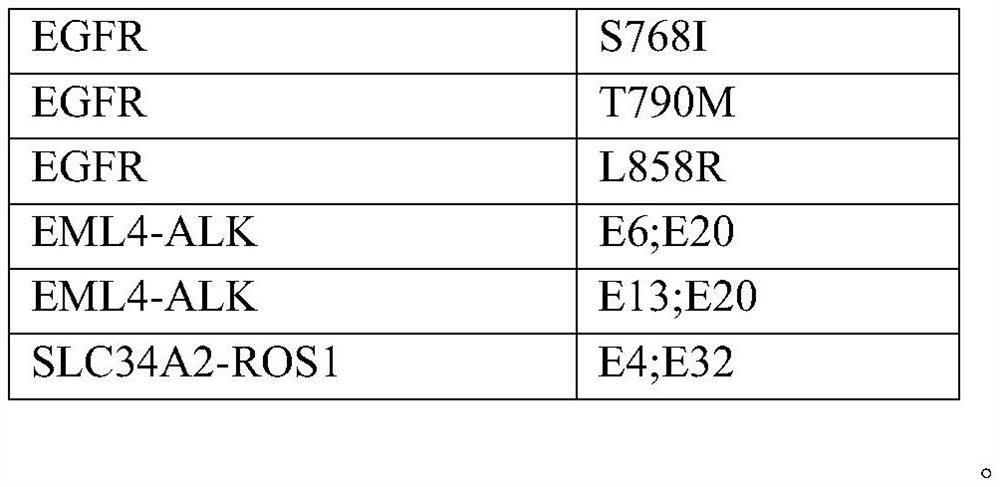
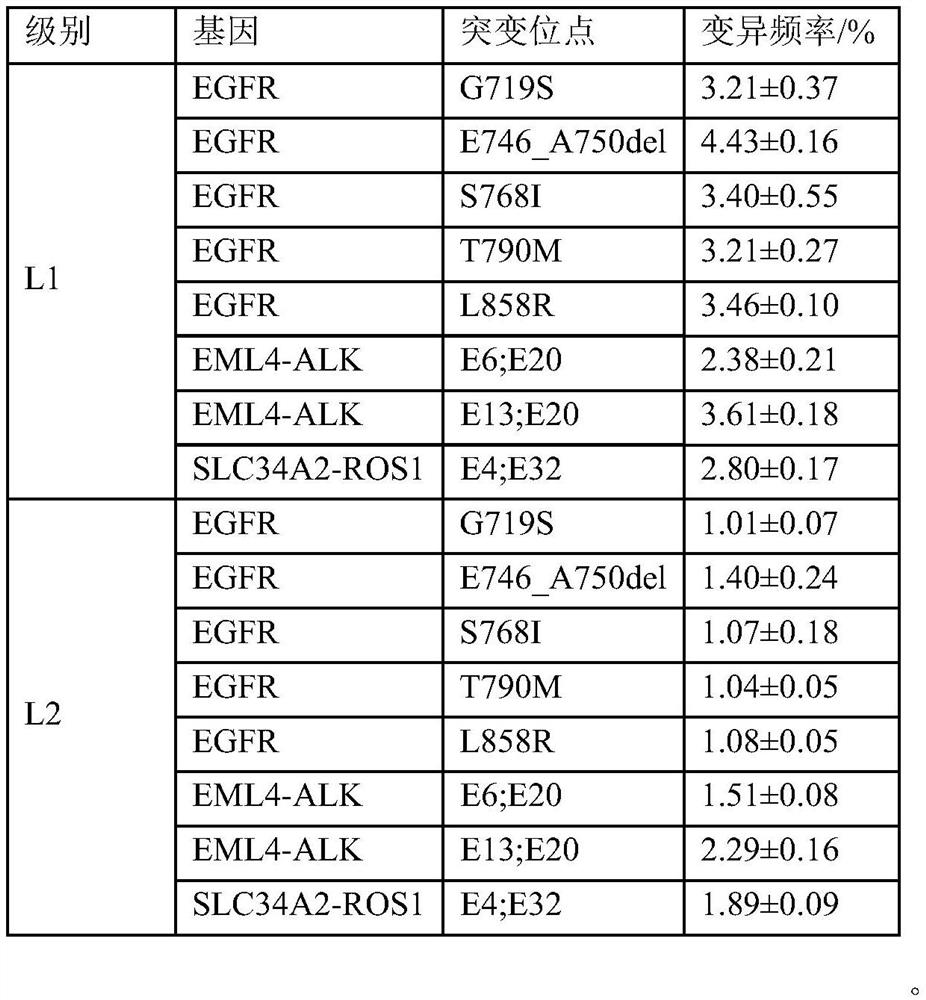
[0057] Purchased 7 tumor cell lines (PC-9, NCI-H1975, KYSE450, SW48, NCI-H2228, NCI-H3122, HCC78) from Nanjing Kebai Biotechnology Co., Ltd. as drug gene detection reagents for lung cancer (reversible end termination sequencing method) Positive reference material for national reference. Purchased 1 normal cell line (BEAS-2B) and 3 tumor cell lines (AMO-1, NCI-H596, NCI-H929) from Nanjing Kebai Biotechnology Co., Ltd. as drug gene detection reagents for lung cancer (reversible terminal termination sequencing Law) The negative reference material of the national reference material. After the cells were cultured, the genomic DNA was extracted and stored at -20°C.
[0058] (2) Negative reference product preparation
[0059] Qubit quantification was performed on BEAS-2B cell line DNA, AMO-1 cell line DNA, NCI-H596 cell line DNA and NCI-H929 cell line DNA to obtain the concentration of cell line DNA (ng / μl). A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com