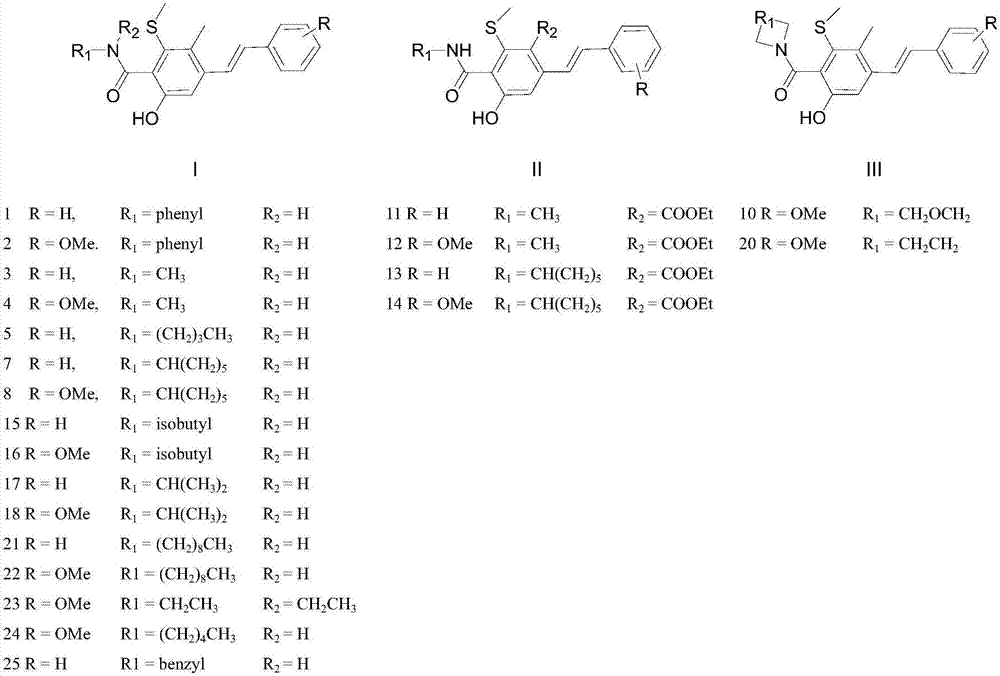
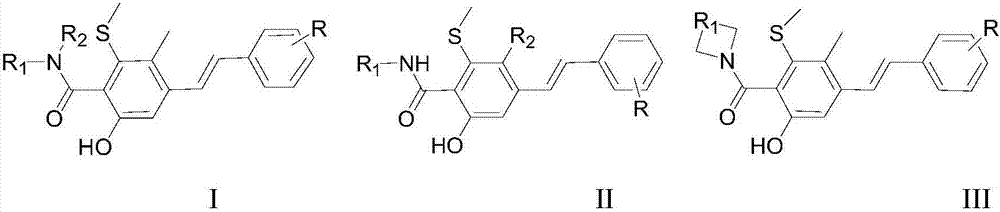
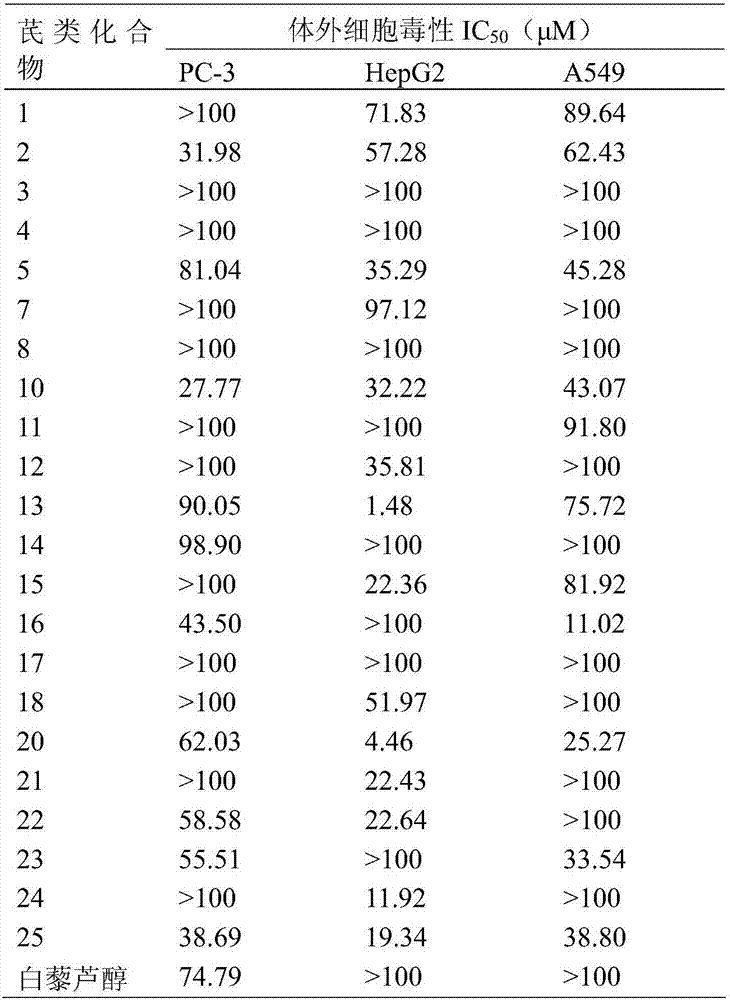
New trans-stilbene-type antitumor agent
A technology for anti-tumor activity and stilbene compounds, which is applied in the field of novel trans-stilbene compounds and anti-tumor agents, and can solve the problems of reduced yield, difficulty in obtaining active stilbene compounds, and difficulty in product separation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Embodiment 1: 10# stilbene compounds are synthesized
[0016] (1) One-pot preparation of α-carbonyl dithioketene: 5 mmol of morpholine was added into a round bottom flask and dissolved in 7 mL of DMF, the temperature was lowered to -10°C using an ice-salt bath, and 5 mmol of diketene was slowly added dropwise. After 10 minutes of dropwise addition, continue to react for 20 minutes and monitor the reaction status using a TLC plate. After the substrate reaction is complete, directly add 10 mmol of weak base potassium carbonate, activate the reaction for 30 minutes, then add 6 mmol of carbon disulfide, the solution turns red, and continue the reaction for 1 hour. Slowly Add 10mmol methyl iodide dropwise, after 30min, slowly rise to room temperature and react for 2-2.5h, use TLC to detect the reaction progress, when the substrate reaction is complete, add distilled water to stop the reaction, and extract with ethyl acetate. The organic layer was collected, washed with water...
Embodiment 2
[0019] Embodiment 2: 13# stilbene compounds are synthesized
[0020] (1) One-pot preparation of α-carbonyl dithioketene: Add 5 mmol of cyclohexylamine to a round bottom flask and dissolve in 7 mL of DMF, use an ice bath to lower the temperature to -10°C, and slowly add 5 mmol of diketene dropwise, After 10 minutes of dropwise addition, continue to react for 20 minutes and monitor the reaction status using a TLC plate. After the substrate reaction is complete, directly add 10 mmol of weak base potassium carbonate, activate the reaction for 30 minutes, then add 6 mmol of carbon disulfide, the solution turns red, and continue the reaction for 1 hour. Slowly Add 10mmol methyl iodide dropwise, after 30min, slowly rise to room temperature and react for 2-2.5h, use TLC to detect the reaction progress, when the substrate reaction is complete, add distilled water to stop the reaction, and extract with ethyl acetate. The organic layer was collected, washed with water three times, and dr...
Embodiment 3
[0023] Embodiment 3: 23# stilbene compounds are synthesized
[0024] (1) One-pot preparation of α-carbonyl dithioketene: 5 mmol of diethylamine was added into a round bottom flask and dissolved in 7 mL of DMF, the temperature was lowered to -10°C using an ice bath, and 5 mmol of diketene was slowly added dropwise. After 10 minutes of dropwise addition, continue to react for 20 minutes and monitor the reaction status using a TLC plate. After the substrate reaction is complete, directly add 10 mmol of weak base potassium carbonate, activate the reaction for 30 minutes, then add 6 mmol of carbon disulfide, the solution turns red, and continue the reaction for 1 hour. Slowly Add 10mmol methyl iodide dropwise, after 30min, slowly rise to room temperature and react for 2-2.5h, use TLC to detect the reaction progress, when the substrate reaction is complete, add distilled water to stop the reaction, and extract with ethyl acetate. The organic layer was collected, washed with water th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com