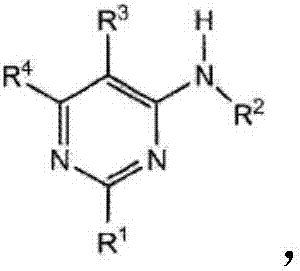
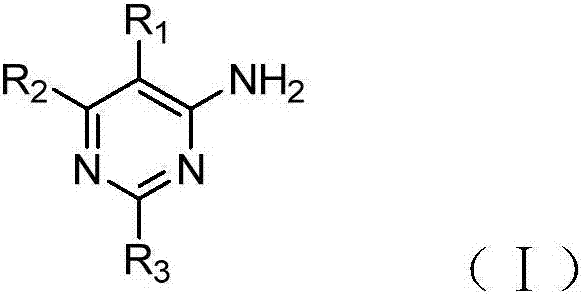
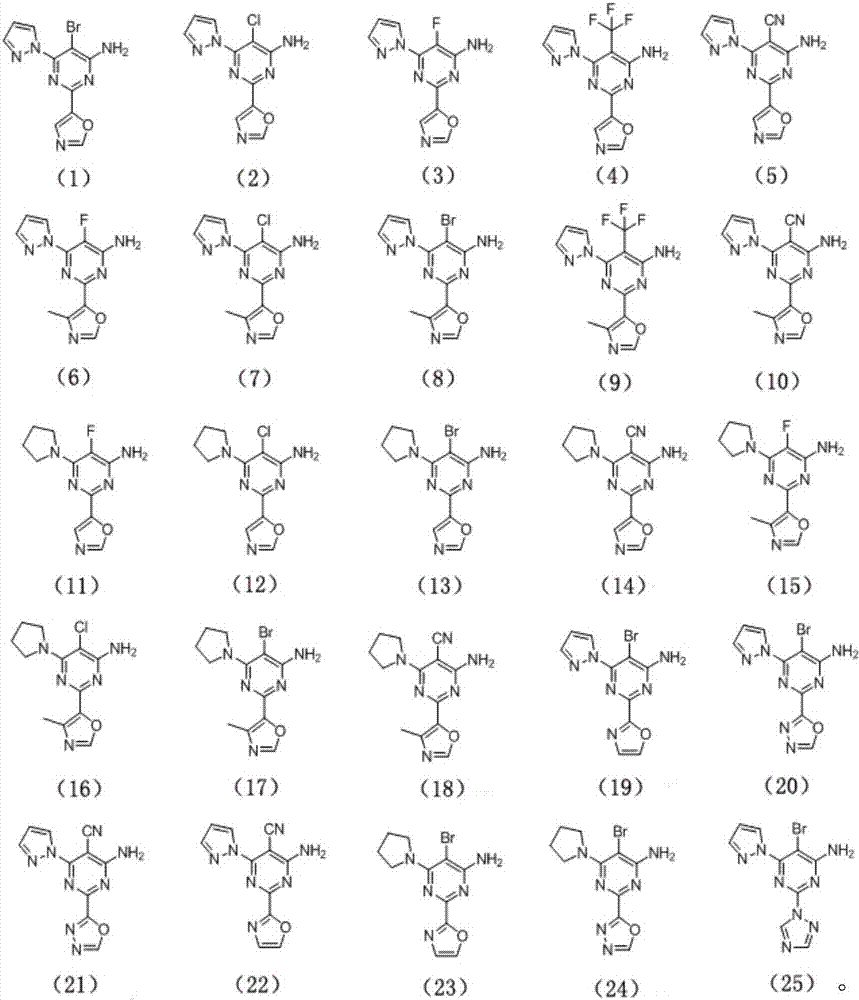
4-aminopyrimidine derivative used as adenosine A2A receptor antagonist and application thereof
A receptor antagonist, aminopyrimidine technology, applied in the field of 4-aminopyrimidine derivatives, can solve the problems of poor pharmacokinetic characteristics, short half-life and high plasma clearance rate of pyrazole compounds, and achieve good pharmacokinetics The effect of features
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] The preparation of compound (1), its structural formula is as follows:
[0073]
[0074] The preparation of compound (1) is implemented according to the above-mentioned synthetic route 1:
[0075] The first step, prepare intermediate (A)
[0076] Suspend 4-amino-2,6-dichloropyrimidine (DCAP, 4g, 24.4mmol) in acetic anhydride (80mL, 860mmol), and heat to reflux under stirring for 4 hours. After the reaction solution was cooled, it was concentrated in vacuo, and the residual acetic anhydride was distilled off after adding toluene. The residue was dissolved in ethyl acetate and water, and 10% NaHCO was added 3 solution until the pH of the solution is 7. The organic layer was washed with saturated brine. After the solvent was recovered, the residue was dissolved in acetic anhydride (40 mL), stirred at 0-5°C for 2 hours, the precipitate was collected by filtration, and dried under vacuum at 40°C to obtain intermediate (A). MS m / z(ESI):206.0[M+1] + .
[0077] Second ...
Embodiment 2
[0088] The preparation of compound (2), its structural formula is as follows:
[0089]
[0090] The preparation of compound (2) is carried out according to the synthetic route 1, and the specific method refers to the example 1. In the 6th step reaction, replace NBS (N-bromosuccinimide) with NCS (N-chlorosuccinimide) for chlorination to obtain compound (2). MS m / z(ESI):263.0[M+1] + , 1 HNMR(400MHz,DMSO-d6)δ8.53(d,1H),8.26(s,1H),7.82(d,1H),7.49(s,1H),7.54(s,2H),6.55(dd,1H ).
Embodiment 3
[0092] The preparation of compound (3), its structural formula is as follows:
[0093]
[0094] Compound (3) was prepared according to Synthetic Route 1. MS m / z(ESI):247.0[M+1] + , 1 HNMR(400MHz,DMSO-d6)δ8.51(d,1H),8.27(s,1H),7.83(d,1H),7.48(s,1H),7.55(s,2H),6.54(dd,1H ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com