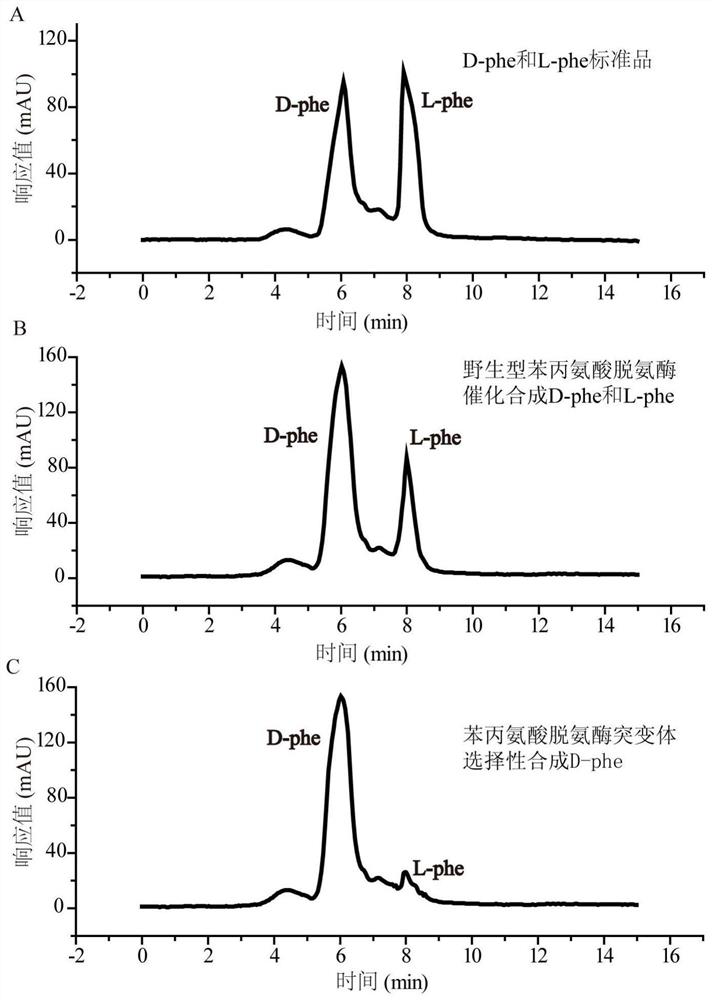
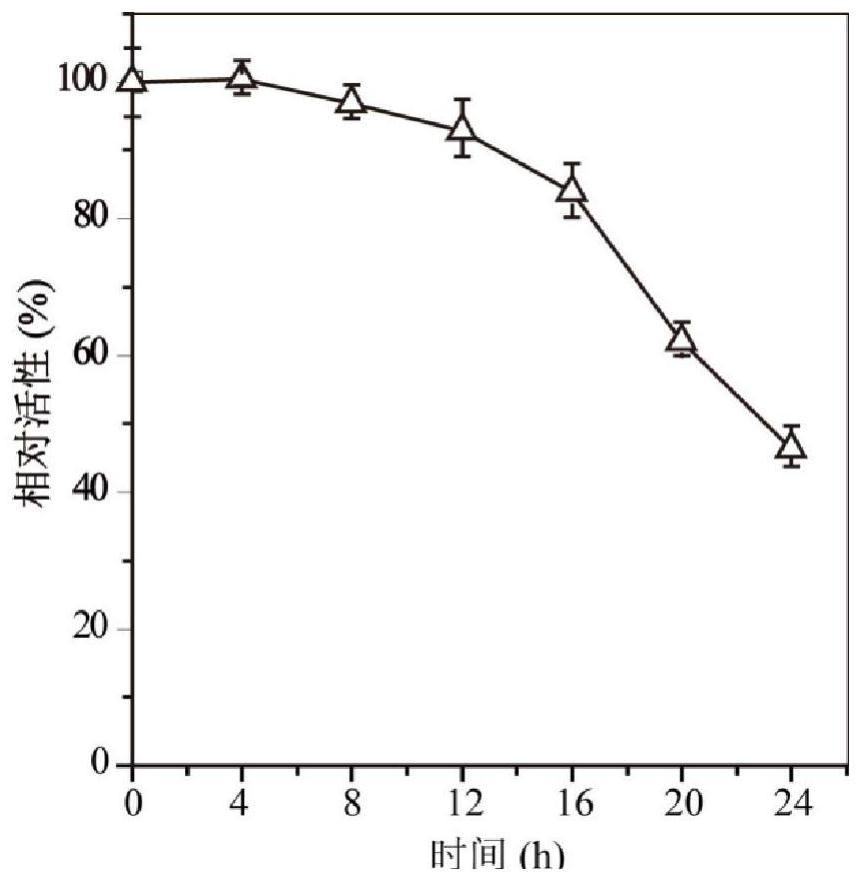
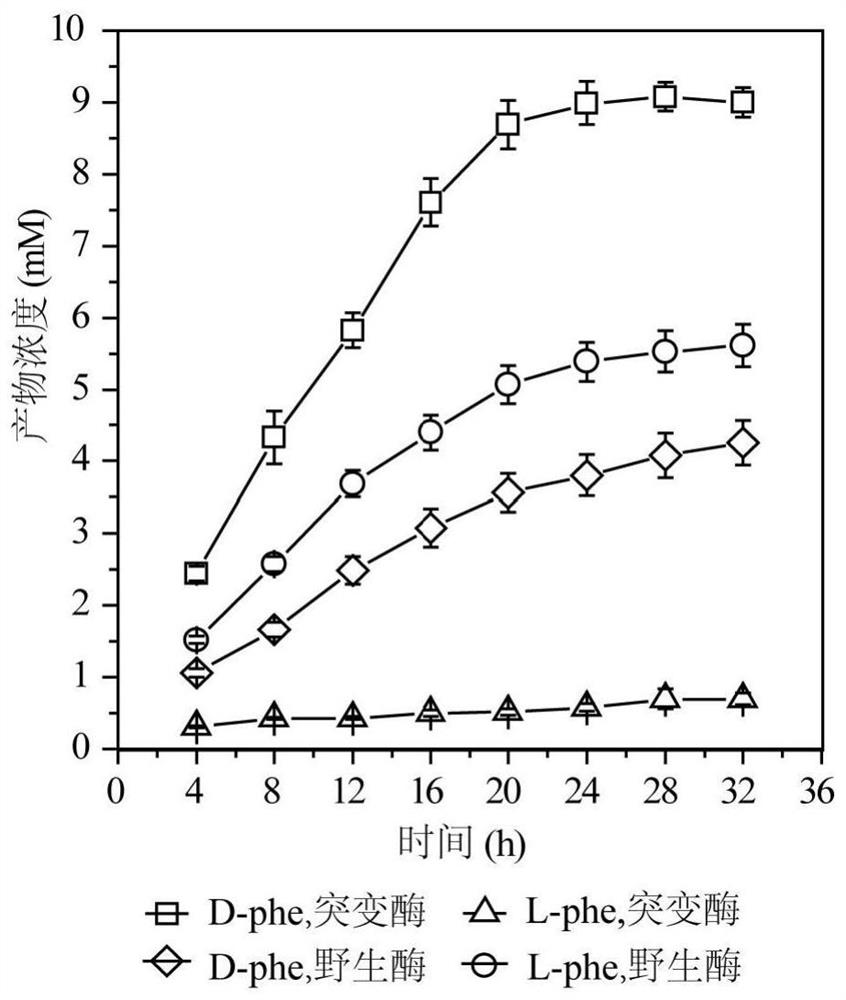
A kind of highly stereoselective phenylalanine deaminase mutant and its application
A technology of phenylalanine deaminase and stereoselectivity, applied in the biological field, can solve the problems of reduced L-aromatic alanine generation ability, difficult separation, low optical purity, etc. Effects of reduced capacity and high thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] (1) With the gene of phenylalanine deaminase derived from prokaryotic organism Anabaena variabilis as a template, design 2 pairs of oligonucleotide primers, and use the unmutated recombinant plasmid pET-28-pal as a template, by PCR Methods Amplified to obtain mutant plasmid pET-28-pal / Gln311Glu / Glu448Thr.
[0022] (2) The sequences of 2 pairs of oligonucleotide primers are as follows:
[0023]
[0024] (3) The amplification reaction system of PCR is:
[0025]
[0026] (4) PCR amplification conditions were: pre-denaturation at 94°C for 1 min, denaturation at 94°C for 1 min, annealing at 56°C for 30 s, extension at 72°C for 7 min, 25 cycles.
[0027] (5) The PCR amplification product was purified and recovered using a DNA purification kit.
Embodiment 2
[0029] (1) The PCR product purified by the DNA purification kit was digested with Dpn I restriction endonuclease, and digested at 37° C. for 1 h. The digestion reaction system is as follows:
[0030]
[0031] (2) The digested product was purified and recovered using a DNA purification kit.
Embodiment 3
[0033] (1) Take the PCR product digested and purified by Dpn I restriction endonuclease, heat shock at 42°C for 60s, transform into Escherichia coli E. / L) on a solid LB plate and cultured at 37°C for 8h. Pick a single colony, insert it into LB liquid medium containing 50mg / L Kan for culture, extract the plasmid, carry out enzyme digestion and PCR verification. The plasmids of positive clones were selected and sent to Shanghai Sangon for sequencing. The correctly sequenced plasmid was heat-shocked at 42°C for 60s and then transformed into E. coli E.coli BL21 competent cells, cultured on LB plates containing Kan (10mg / L) resistance at 37°C for 8h, and the positive transformants were selected, namely phenylpropanoid Amino acid deaminase mutant producing bacteria.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com