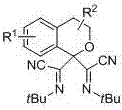
Bis alpha-cyanoimino substituted isochroman compound and synthetic method thereof
A technology of cyanoimine and isochroman, which is applied in the field of bis-cyanoimine replacing isochroman compounds and its synthesis, can solve the problems of complex substrates, toxic cyanide, cumbersome operation, etc., and achieve high The effect of reactivity, simple raw materials, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
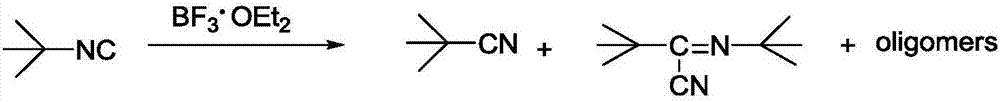
Method used
Image
Examples
Embodiment 1
[0033] Example 1: N, N'-di-tert-butyl isochroma-1,1-bis(formamimidino) dicyanide
[0034] N, N'-di-tert-butyl isochroma-1,1-bis(carboxamidino) dicyanide adopts the following steps: 1. Add 9.4 grams of isochroman, 17.4 grams of tert-butyl isocyanide, 1.8 grams of Silver trifluoromethanesulfonate, 15.9 g of 2,3-dichloro-5,6-dicyano-1,4-benzoquinone, 700 ml of chlorobenzene, heated to 80°C. Use thin layer chromatography to track the reaction until the reaction raw materials disappear; ② After the reaction, extract the product with ethyl acetate, wash with saturated brine, and remove the solvent with a rotary evaporator after drying to obtain a crude product; ③ Use a column layer for the crude product Analysis (petroleum ether: ethyl acetate=100:1) purification, obtains 14.71 grams of N, N'-di-tert-butyl isochromatic-1,1-bis(formamidinyl) dicyanide, its structural formula is: The yield was 60%. Melting point: 114°C.
[0035] IR(KBr,cm -1 ):2979,2216,1643,1476,1464,1208,914,75...
Embodiment 2
[0040] Example 2: N, N'-di-tert-butyl-5-methylisochroma-1,1-bis(formamimidino) dicyanide
[0041] N, N'-di-tert-butyl-5-methyl isochromatic-1,1-bis(formamimidino) dicyanide adopts the following steps: 1. add 10.4 grams of 5-methyl in a 1000 ml reaction kettle Isochroman, 17.4 g tert-butylisonitrile, 3.6 g silver triflate, 15.9 g 2,3-dichloro-5,6-dicyano-1,4-benzoquinone, 700 ml chlorobenzene, heating to 80°C. Use thin layer chromatography to track the reaction until the reaction raw materials disappear; ② After the reaction, extract the product with ethyl acetate, wash with saturated brine, and remove the solvent with a rotary evaporator after drying to obtain a crude product; ③ Use a column layer for the crude product Analysis (petroleum ether: ethyl acetate=100:1) purified to obtain 19.12 grams of N, N'-di-tert-butyl-5-methylisochromatic-1,1-bis(formamimidino) dicyanide, Its structural formula is: The yield was 75%. Melting point: 104°C.
[0042] IR(KBr,cm -1 ):2976,2...
Embodiment 3
[0047] Example 3: N, N'-di-tert-butyl-7-phenyl isochroma-1,1-bis(formamimidino) dicyanide
[0048] N, N'-di-tert-butyl-7-phenyl isochromatic-1,1-bis(formamimidino) dicyanide adopts the following steps: 1. add 14.7 grams of 7-benzene in a 1000 ml reaction kettle Isochroman, 17.4 g tert-butylisonitrile, 2.7 g silver triflate, 15.9 g 2,3-dichloro-5,6-dicyano-1,4-benzoquinone, 700 ml chlorobenzene, Heat to 80°C. Use thin layer chromatography to track the reaction until the reaction raw materials disappear; ② After the reaction, extract the product with ethyl acetate, wash with saturated brine, and remove the solvent with a rotary evaporator after drying to obtain a crude product; ③ Use a column layer for the crude product Analysis (petroleum ether: ethyl acetate = 100:1) purification, to obtain 23.87 grams of N, N'-di-tert-butyl-7-phenylisochromatic-1,1-bis(formamimidino) dicyanide , whose structural formula is: The yield was 80%. Melting point: 125°C.
[0049] IR(KBr,cm -1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com