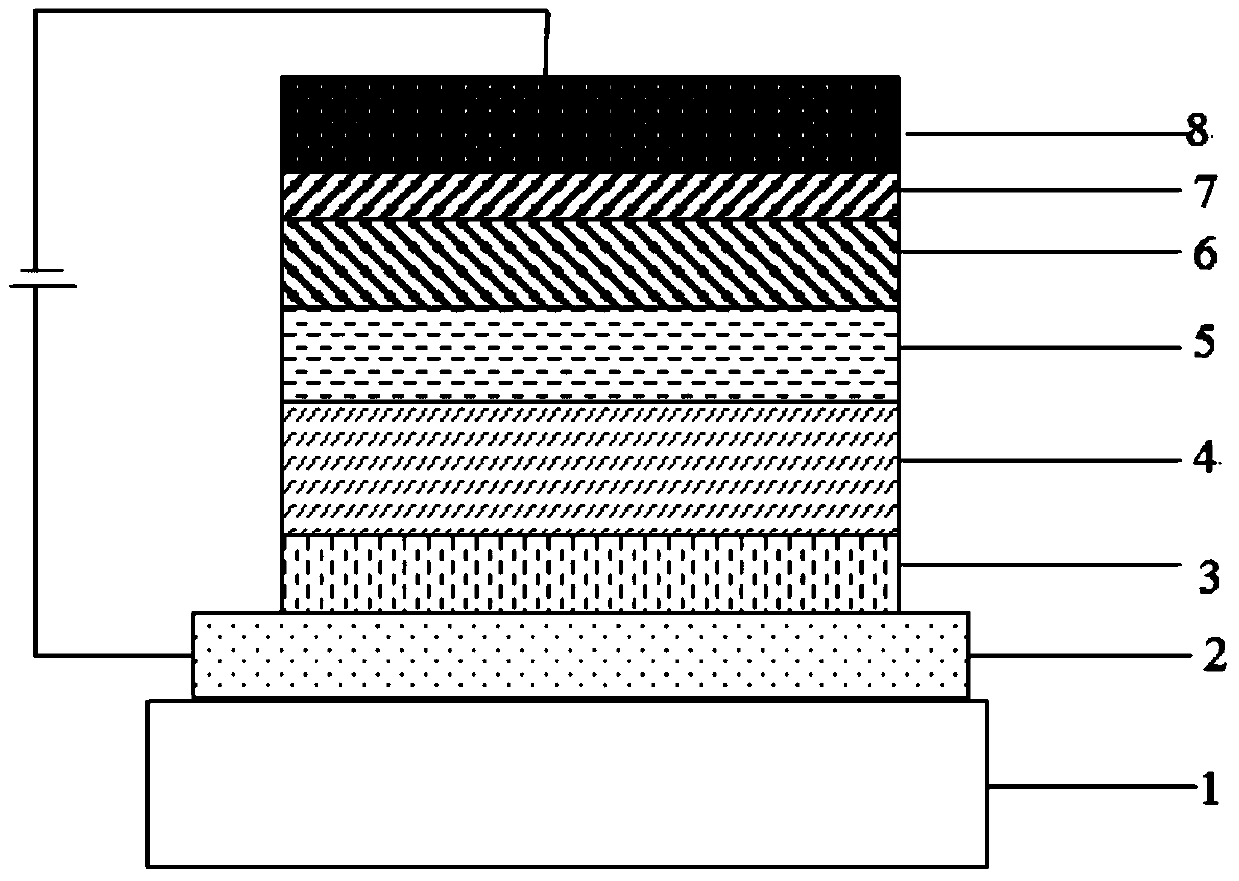
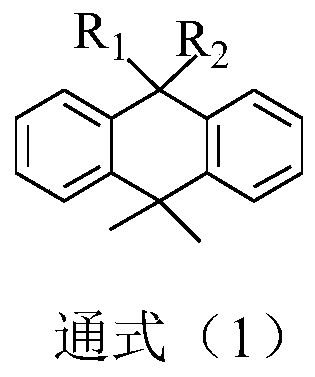
A kind of photoelectric material containing dimethyl anthracene structure and its application
A technology of optoelectronic materials, dimethyl anthracene, applied in luminescent materials, hydrocarbon production from oxygen-containing organic compounds, circuits, etc., can solve problems such as different problems, and achieve the effects of avoiding aggregation, reducing device voltage, and improving film formation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] The synthesis of embodiment 1 compound 2:
[0047]
[0048] synthetic route:
[0049]
[0050] In a 250ml four-necked flask, under nitrogen atmosphere, add 16.2g 4-(4-bromo-phenyl) dibenzofuran (0.05mol) and 1.44g Mg powder (0.06mol), 60ml tetrahydrofuran, heat to reflux for 4 hour, the reaction is complete, and the format reagent is generated;
[0051] Dissolve 11.1g of 10,10-dimethylanthrone (0.05mol) in 50ml of tetrahydrofuran, add the above-mentioned Grignard reagent dropwise, react at 60°C for 24 hours, a large amount of white precipitate is formed, and finally add saturated NHCl 4 Convert the Grignard salt into alcohol; after the reaction, extract with ether, dry and rotary evaporate, and purify on a silica gel column with a mixed solvent of petroleum ether: dichloromethane (3:2) to obtain a slightly yellow solid tertiary alcohol (85% yield) ; using DEI-MS to identify the compound, formula C 34 h 26 o 2 , detection value [M+1] + = 466.93, calculated va...
Embodiment 2
[0054] The synthesis of embodiment 2 compound 4:
[0055]
[0056] synthetic route:
[0057]
[0058] Prepared according to the synthetic method of compound 1 in Example 1, the difference is that 4-(4-bromo-phenyl) dibenzofuran is replaced by 6-(4-bromo-phenyl)naphthobenzofuran, and biphenyl instead of benzene;
[0059] Using DEI-MS to identify the compound, formula C 50 h 36 O, detection value [M+1] + =653.13, calculated value 652.28.
Embodiment 3
[0060] The synthesis of embodiment 3 compound 28:
[0061]
[0062] synthetic route:
[0063]
[0064] Prepared according to the synthetic method of compound 1 in Example 1, the difference is that 1-(4-bromo-phenyl)-9,9-dimethylfluorene is used instead of 4-(4-bromo-phenyl)dibenzofuran , replace benzene with biphenyl;
[0065] Using DEI-MS to identify the compound, formula C 49 h 40 , detection value [M+1] + =629.15, calculated value 628.31.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com