Application of vegf monoclonal antibody combined with nano-paclitaxel in the preparation of drugs for the treatment of cancerous abdominal adhesions
A paclitaxel compound and paclitaxel technology, which are applied in the field of VEGF monoclonal antibody combined with nano-paclitaxel composition, can solve the problems of multiple complications, poor curative effect, increased mortality, etc., and achieve the effect of obvious symptom improvement and less toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
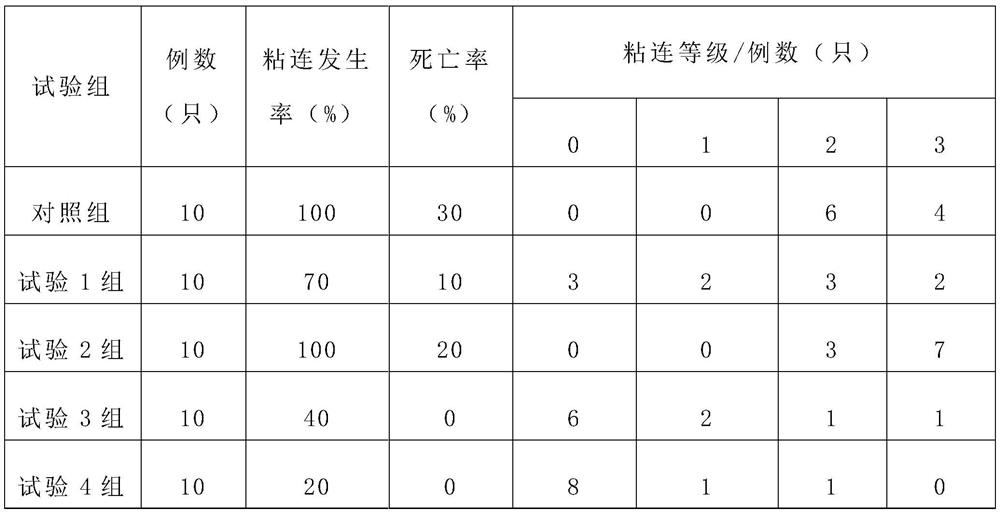
[0020] Example 1 The therapeutic effect of VEGF monoclonal antibody combined with nano-paclitaxel on postoperative abdominal adhesions in mice
[0021] 1.1 Animals: Kunming mice, half male and half male
[0022] 1.2 Establishment of abdominal injury model
[0023] Animals were weighed before the operation, and pentobarbital sodium was given according to the dosage of 38 mg / kg. After complete anesthesia, under aseptic conditions, the abdominal incision was made in the middle of the lower abdomen. The sides of the intestinal canal were repeatedly rubbed with gauze 30 times, and the parietal peritoneum on the right side of the midline was repeatedly clamped with hemostatic forceps 30 times, and then the peritoneum was sutured with absorbable sutures, and finally the therapeutic drugs were slowly injected into the abdominal cavity before the sutures were ligated , and then ligated and sutured, closing the abdomen layer by layer. After fasting for 12 hours, the rats were fed in s...
Embodiment 2
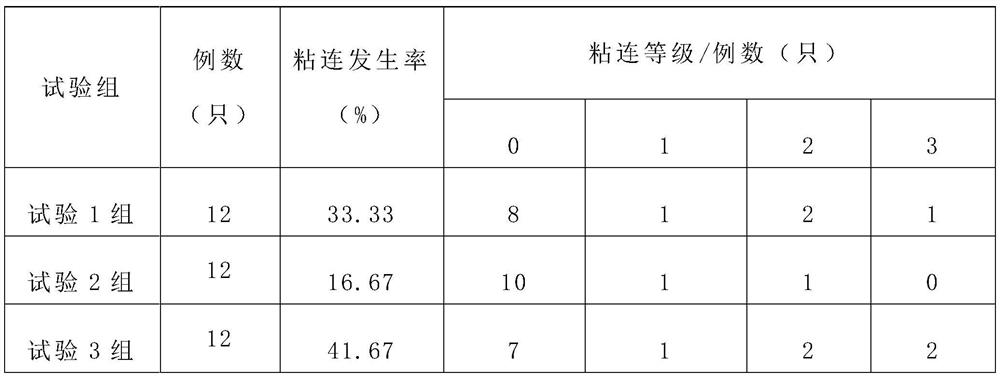
[0033] Example 2 The therapeutic effect of VEGF monoclonal antibody formed by different VEGF monoclonal antibodies combined with nano-paclitaxel on postoperative abdominal adhesions in mice
[0034] The experimental animals used in this example, the adhesion evaluation criteria and the establishment of the abdominal cavity injury model are the same as those in Example 1.
[0035] 2.1 Grouping situation
[0036] 72 Kunming mice were randomly selected into 6 groups, 12 in each group, half male and half female. The treatment drug of test group 1 was bevacizumab combined with nano-paclitaxel; the treatment drug of test group 2 was ranibizumab combined with nano-paclitaxel; test 3 The treatment drug of group 4 was sunitinib combined with nano-paclitaxel; the drug of test group 4 was ranibizumab combined with nano-paclitaxel; the drug of test group 5 was pegatanib combined with nano-paclitaxel; the drug of test group 6 was VEGF rabbit IgG The weight ratio of anti-combined nano-pacl...
Embodiment 3
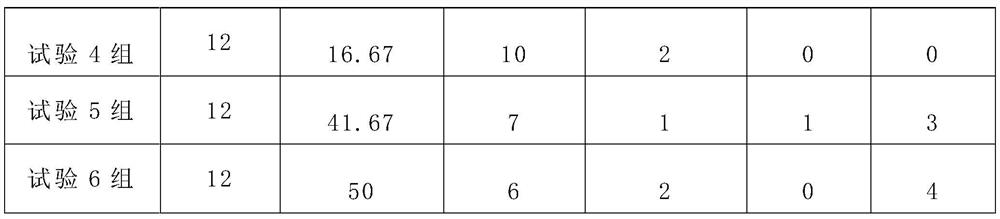
[0042] Example 3 Therapeutic effect of VEGF monoclonal antibody combined with nano-paclitaxel prepared from different weight ratios of VEGF monoclonal antibody and nano-paclitaxel on postoperative abdominal adhesions in mice
[0043]The experimental animals used in this example, the adhesion evaluation criteria and the establishment of the abdominal cavity injury model are the same as those in Example 1.
[0044] 3.1 Grouping situation
[0045] 80 Kunming mice were randomly selected into 8 groups, 10 in each group, half male and half male, and ranibizumab was combined with nano-paclitaxel to investigate the therapeutic effect of the compound obtained with different ratios of the two raw materials on abdominal adhesions. The drugs were diluted with 150mmol / L normal saline according to the volume of 1:100 before the test.
[0046] 3.2 Test results
[0047] Table 3 Effects of VEGF monoclonal antibody and nano-paclitaxel in different weight ratios on the therapeutic effect of VE...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com