Novel heterocyclic derivative with CDK (cyclin-dependent kinase) 4/6 and HDAC (histone deacetylase) inhibitory activities
A CH2, heteroaromatic ring technology, applied in the field of novel heterocyclic derivatives and pharmaceutical compositions thereof, can solve the problems of enhanced deacetylation, increased DNA and histone attraction, unfavorable tumor suppressor gene expression and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0021] The starting materials used in the preparation of the compounds of the present invention are known, can be prepared according to known methods, or are commercially available.
[0022] The invention also relates to novel intermediates and / or starting materials. Particular preference is given to reaction conditions and novel intermediates which are the same or similar to those mentioned in the examples.
[0023] Both intermediates and final products can be worked up and / or purified according to conventional methods including pH adjustment, extraction, filtration, drying, concentration, chromatography, trituration, crystallization, and the like.
[0024] In addition, the compounds of the present invention can be prepared by various methods known in the art or variations on the methods described herein.
[0025] The following examples are only used to illustrate the present invention and do not limit the present invention in any way.
Embodiment 1
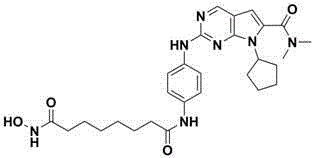
[0026] Example 1 8-((4-((7-cyclopentyl-6-(dimethylcarbamoyl)-7H-pyrrole[2,3-d]pyrimidin-2-yl)amino)phenyl)amino) - Preparation of methyl 8-oxooctanoate
[0027]
[0028] Step 1.1: Preparation of 8-((4-aminoaniline)amino)-8-oxooctanoic acid methyl ester
[0029]
[0030]Add monomethyl suberate (3.97 g, 21.3 mmol), p-phenylenediamine (2.31 g, 21.3 mmol), DMF 30 mL and DIPEA (13 mL, 74.4 mmol) into a 500 mL single-necked bottle. Stir at room temperature for 5 minutes. Add EDCI (4.5 g, 23.4 mmol) and stir overnight at room temperature. After the reaction was completed, ammonium chloride aqueous solution (30 g ammonium chloride, 300 mL water) was slowly added dropwise. After the drop was completed, the mixture was stirred at room temperature for 2 h, filtered, and the solid was collected and vacuum-dried to obtain 3.19 g of a pink solid, with a yield of 51%. 1 H NMR (400 MHz, DMSO- d 6 ) δ 9.42 (s, 1H), 7.19 (d, J = 8.5 Hz, 2H), 6.47 (d, J = 8.5 Hz, 2H), 4.82 (s, 2H)...
Embodiment 2
[0034] Example 2 N-1-(4-((7-cyclopentyl-6-(dimethylcarbamoyl)-7H-pyrrole[2,3-d]pyrimidin-2-yl)amino)phenyl)- Preparation of N-8-Hydroxyaminooxyoctanoic Acid Amide
[0035]
[0036] Add 8-((4-((7-cyclopentyl-6-(dimethylcarbamoyl)-7H-pyrrole[2,3-d]pyrimidin-2-yl)amino)benzene Base)amino)-methyl 8-oxooctanoate (250 mg, 0.47 mmol), hydroxylamine aqueous solution (50%, 2 ml), methanol 5 mL, heated to reflux, and reacted overnight. After the reaction, the solvent was removed, and the residue was purified by column chromatography to obtain 199 mg, yield = 79.4%. 1 H NMR (400 MHz, DMSO- d 6 ) δ 10.34 (s, 1H), 9.74 (s, 1H), 9.44 (s, 1H), 8.72 (s, 1H), 7.81 – 7.65 (m, 2H), 7.60 – 7.44 (m, 2H), 6.57 ( s, 1H), 4.83 – 4.64 (m, 1H), 3.06 (s, 6H), 2.47 (m, 2H), 2.27 (t, J = 7.4 Hz, 2H), 2.20 (t, J = 7.3 Hz, 1H), 1.97 (dq, J = 14.6, 7.4 Hz, 5H), 1.73 – 1.45 (m, 6H), 1.34 – 1.26 (m, 4H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com