A pharmaceutical composition for preventing and treating diarrhea in weaned piglets and its preparation method and application
A technology for weaning piglet diarrhea and composition, which is applied in the field of pharmaceutical composition for preventing and treating weaning piglet diarrhea and its preparation, which can solve the problems of short half-life, large side effects, inconvenient use, etc., and achieve the promotion of body immune function, low cost, and convenient material selection Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
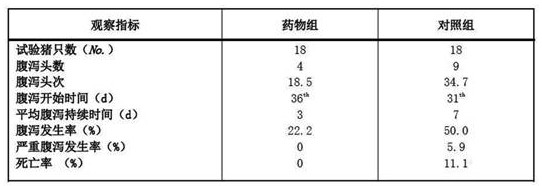
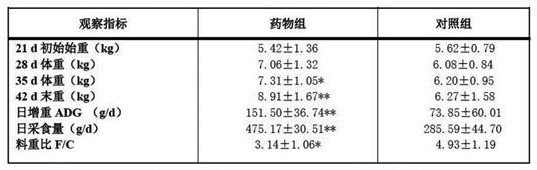
Examples
Embodiment 1
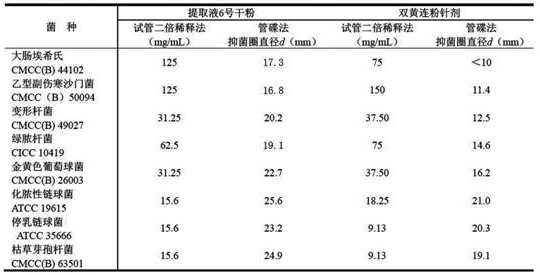
[0045] Embodiment 1 specific anti E. Coli -IgY, anti-TGE-PED-PoRV-IgY egg yolk antibody and preparation of corresponding egg yolk powder
[0046] (1) Preparation of different types of highly immunogenic immune antigens and immunization schemes
[0047] ① The immunogen is Escherichia coli bacterial protein antigen immune substance
[0048] Select Escherichia coli ( E. coli, O157:K88, CVCC1489, purchased from China Veterinary Microbiological Culture Collection Management Center). The culture of the main pathogenic bacteria is preferably subcultured on blood agar slant medium, cultivated at 37 °C for 24 hours, and there is no miscellaneous bacteria under the staining microscope to obtain pure species. The main pathogenic bacteria were inoculated in LB broth medium (containing 5-10% calf serum and 1-2% glucose) for expansion culture, and placed in a constant temperature shaking incubator at 37 ℃ (150-200 r / min ), cultured for 24 h, and the bacterial cells were collected by c...
Embodiment 2
[0058] Example 2 The preparation of probiotic yeast dry powder, the steps are as follows:
[0059] (1) Preparation of medium:
[0060] Liquid medium: the composition includes glucose 50 g·L -1 , yeast extract 2.0 g L -1 , Wort 120 mL / L, MgSO 4 0.5 g L -1 , FeSO 4 0.1g L -1 , urea 1.0 g·L -1 . Adjust the pH to 6.0, sterilize at 121°C for 30 min, dispense into Erlenmeyer flasks, and set aside.
[0061] Slant and plate medium (g L -1 ): Ingredients include glucose 50 g·L -1 , yeast extract 2.0 g L -1 , Wort 120 mL / L, MgSO 4 0.5 g L -1 , FeSO 4 0.1g L -1 , urea 1.0 g·L -1 , agar 20 g·L -1 . Adjust the pH to 6.0, sterilize at 121°C for 20 min, pour into the plate, and set aside.
[0062] (2) Yeast culture:
[0063] Saccharomyces cerevisiae( Saccharomyces cerevisiae , purchased from China Veterinary Microbiological Strain Preservation Management Center, feed yeast, strain number 2.1011), pick out a ring of relatively plump yeast lawn from the slant storage seed, ...
Embodiment 3
[0067] Example 3 The preparation method of the dry powder of the compound Chinese medicine extract, wherein the proportioning ratio of each single Chinese medicine is as follows:
[0068] The traditional Chinese medicine composition consists of the following components in parts by weight:
[0069] 10-30 parts of Sophora flavescens, 10-30 parts of Burnet, 10-20 parts of Coptidis, 10-20 parts of Phellodendron 10-20, 10-20 parts of gall, 10-20 parts of dandelion, 10-20 parts of Dijincao, 5 parts of Houttuynia cordata -15 parts, 5-15 parts of agrimony, 5-15 parts of purslane, 5-15 parts of plantain, 2-15 parts of barley, 2-15 parts of yarrow, 5-15 parts of licorice.
[0070] The preparation method of Chinese medicine compound extract dry powder is:
[0071] (1) Pulverize each single traditional Chinese medicine through a superfine powder pulverizer, and pass through a 100-120 mesh sieve;
[0072] (2) Formulate and mix the superfine powder single-flavor Chinese medicines obtained...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com