Method for rapidly purifying and isolating anti-actin F(ab')2 and Fc fragments
A technology for purification and separation of proteins, applied in chemical instruments and methods, peptide preparation methods, immunoglobulins, etc., can solve the problems of increased experimental costs, more reagent consumables, and the impact of experimental analysis, so as to reduce experimental costs and instrument maintenance The effect of cost reduction, reduced experiment use time, and simple operation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
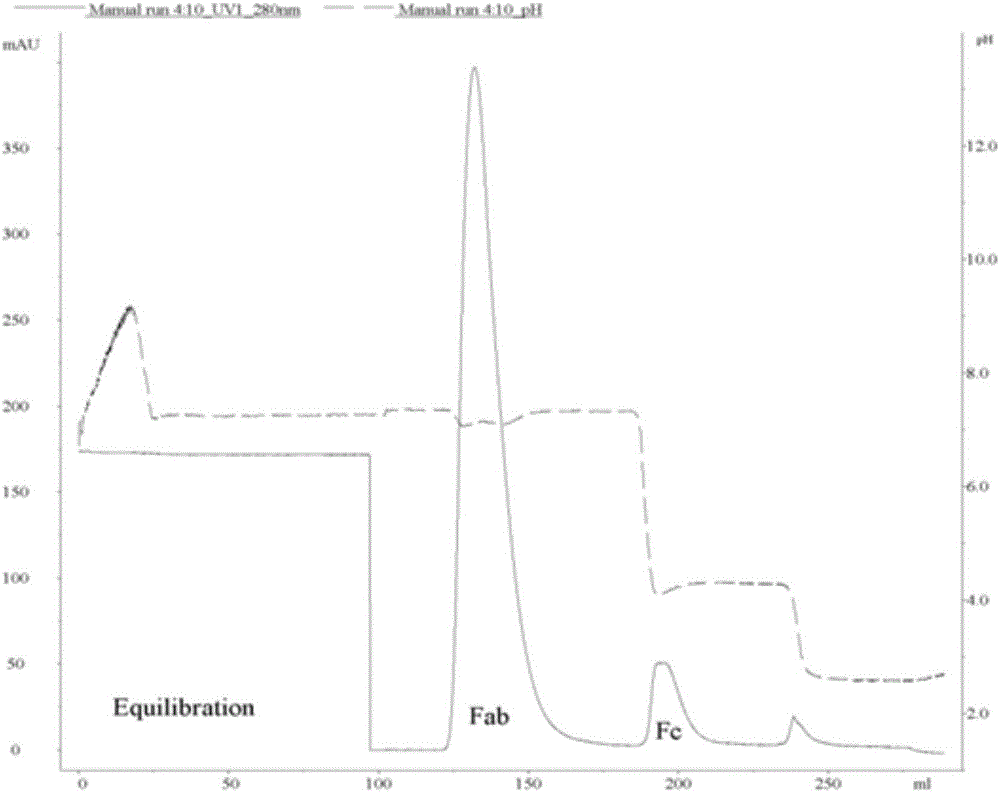
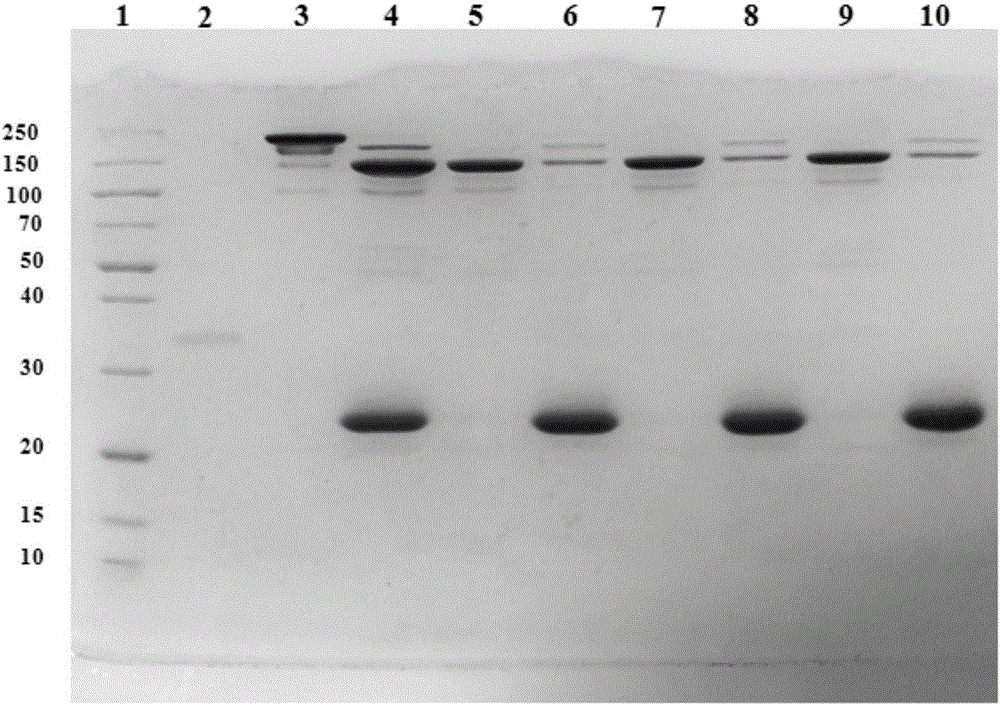
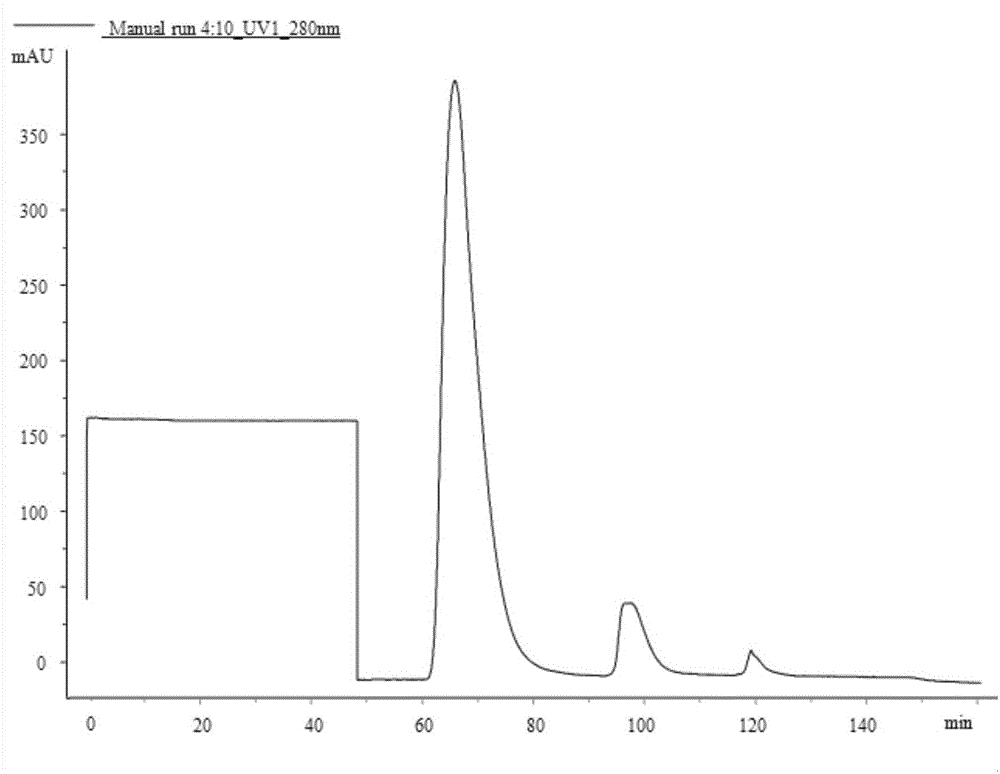
[0042] This protocol allows samples to be obtained within 3 hours and subsequent assays can be performed immediately, and antibody fragments are prepared according to the protocol. 12% separation gel was used to analyze and verify the samples after enzymatic hydrolysis and the samples separated by magnetic beads three times. figure 2 SDS-PAGE results of antibody fragments. In the figure, Lane1 is molecular weight marker (Thermo), Lane2 is endonuclease, Lane3 is intact IgG, Lane4 is digested IgG, Lane5, 7, and 9 are F(ab') 2 Fragments, Lane6, 8, 10 are Fc fragments. Comparing Lane5 and Lane 6, it can be seen that the separation effect of this scheme is really reliable, F(ab') 2 Fragments can be completely separated from Fc fragments. At the same time, F(ab') can be seen by repeated use of magnetic beads 2 The separation effect is still ideal (Lane5, 7, 9), while the Fc fragments are all captured by the magnetic beads (Lane6, 8, 10).
[0043] It can be seen that this metho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com