Preparation method for morphology-controllable hematite for degrading environmental pollutants
A technology of environmental pollutants and hematite, which is applied in the direction of iron oxide, iron oxide/iron hydroxide, etc., can solve the problems of high energy consumption and low yield of synthetic products, and achieve simple production process, fast reaction rate and excellent effect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
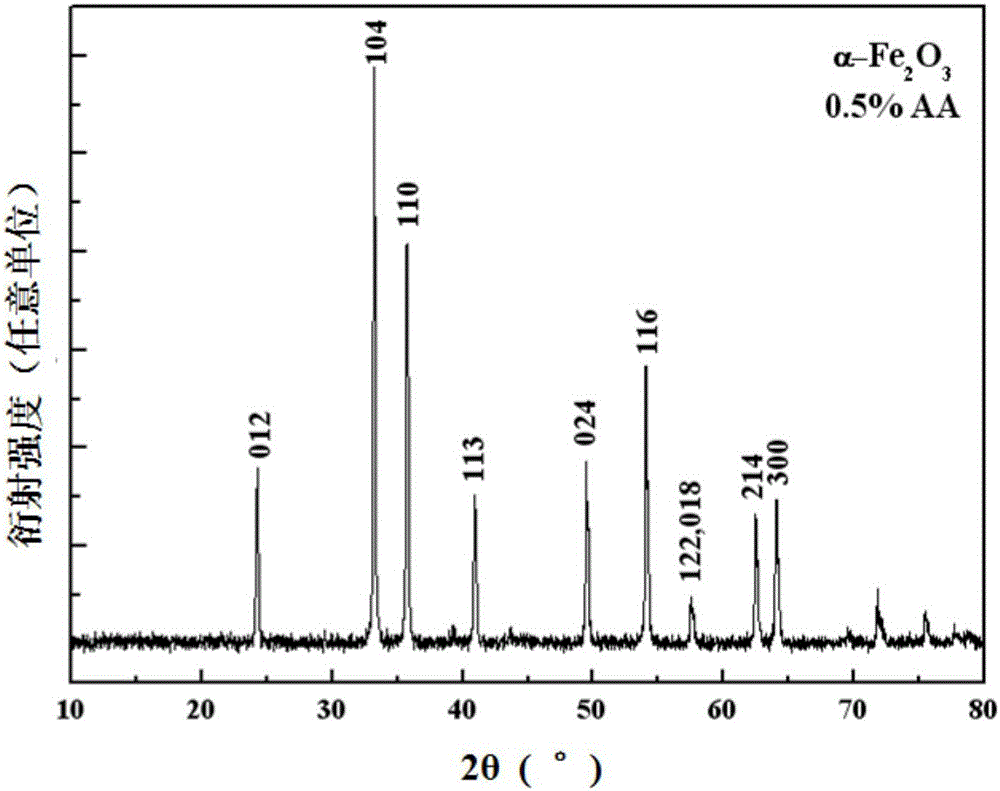
[0034] Embodiment 1 (0.5%AA reducing condition)
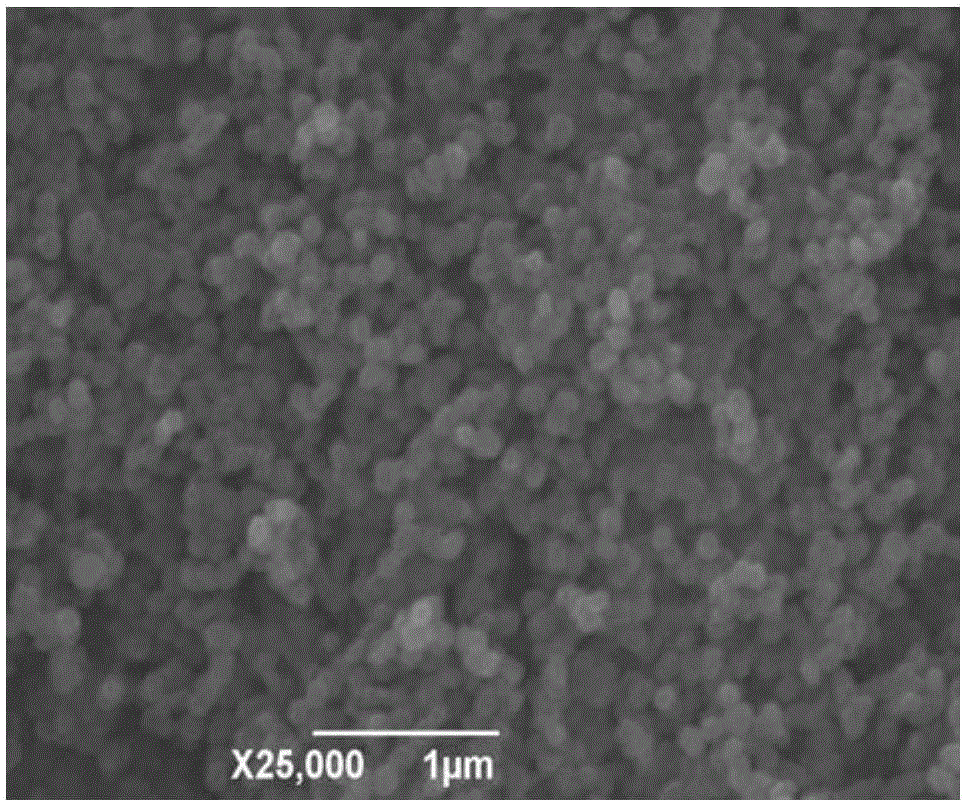
[0035] First, weigh 6.750g FeCl 3 ·6H 2 O in a 500ml plastic bottle, add about 200ml distilled water to prepare FeCl 3 The solution is ready for use. At the same time, weigh 12.000g NaOH and dissolve it in 50ml distilled water to prepare a 6mol / L NaOH solution for use; 3 Add 6mol / L NaOH solution drop by drop in the solution until the pH in the system is about 7, the 3+ The molar ratio is 0.5%, and 0.044g reduced ascorbic acid is added; then, the pH value of the system is adjusted back to 7 with 0.1mol / L NaOH solution, and the volume is adjusted to 250ml with distilled water to make the final Fe of the system 3+ The concentration is 0.1mol / L; finally, after stirring evenly, the aforementioned product is aged in an oil bath heated to 100°C for 5 hours, and after cooling, the solid precipitate is separated by centrifugation, and the solid precipitate is washed several times with deionized water When the conductivity is less th...
Embodiment 2
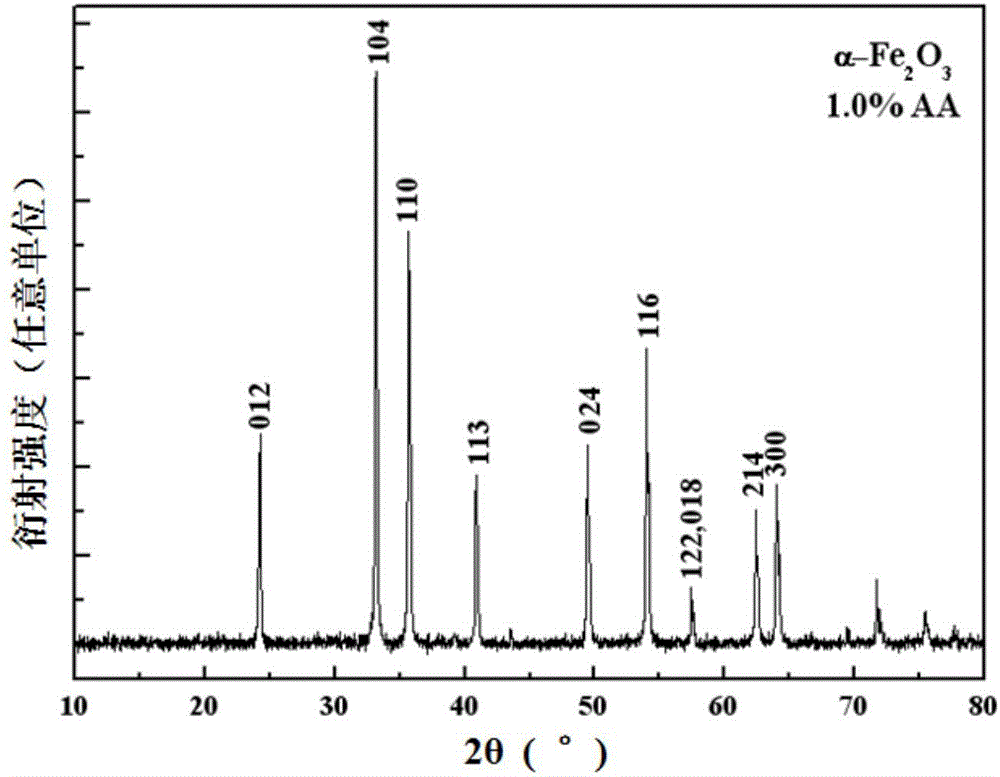
[0037] Embodiment 2 (1.0%AA reduction condition)
[0038] First, weigh 6.750g FeCl 3 ·6H 2 O in a 500ml plastic bottle, add about 200ml distilled water to prepare FeCl 3 The solution is ready for use. At the same time, weigh 12.000g NaOH and dissolve it in 50ml distilled water to prepare a 6mol / L NaOH solution for use; 3 Add 6mol / L NaOH solution drop by drop in the solution until the pH in the system is about 7, the 3+ The molar ratio is 1.0%, and 0.088g reduced ascorbic acid is added; then, the pH value of the system is adjusted back to 7 with 0.1mol / L NaOH solution, and the volume is adjusted to 250ml with distilled water to make the system final Fe 3+ The concentration is 0.1mol / L; finally, after stirring evenly, the aforementioned product is aged in an oil bath heated to 100°C for 5 hours, and after cooling, the solid precipitate is separated by centrifugation, and the solid precipitate is washed several times with deionized water When the conductivity is less than 20μ...
Embodiment 3
[0040] Embodiment 3 (2.0%AA reduction condition)
[0041] First, weigh 6.750g FeCl 3 ·6H 2 O in a 500ml plastic bottle, add about 200ml distilled water to prepare FeCl 3 The solution is ready for use, meanwhile, take 12.000g NaOH and dissolve it in 50ml distilled water to prepare a 6mol / L NaOH solution for use; then, add FeCl under magnetic stirring condition 3 Add 6mol / L NaOH solution drop by drop in the solution until the pH in the system is about 7, the 3+ The molar ratio is 2.0%, and 0.176g of reduced ascorbic acid is added; then, the pH value of the system is adjusted back to 7 with 0.1mol / L NaOH solution, and the volume is adjusted to 250ml with distilled water to make the final Fe 3+ The concentration is 0.1mol / L; finally, after stirring evenly, the aforementioned product is aged in an oil bath heated to 100°C for 12 hours, and the solid precipitate is separated by centrifugation after cooling, and the solid precipitate is washed several times with deionized water W...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com