Brexpiprazole methyl alcohol compound, crystal form A and preparation method and application
A technology for epipiprazole methyl and alcoholate, applied in the field of epipiprazole methylate, can solve the problems of cumbersome preparation process, unsuitable for industrial production, poor crystal form solubility, etc., and achieves good sustained-release effect and high marketability Good chemical prospect and easy dissociation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
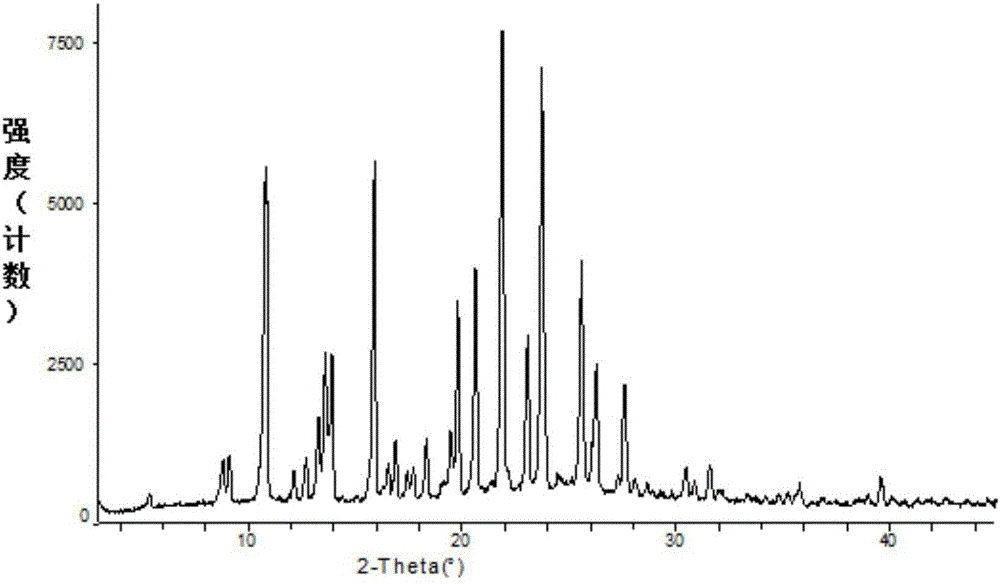
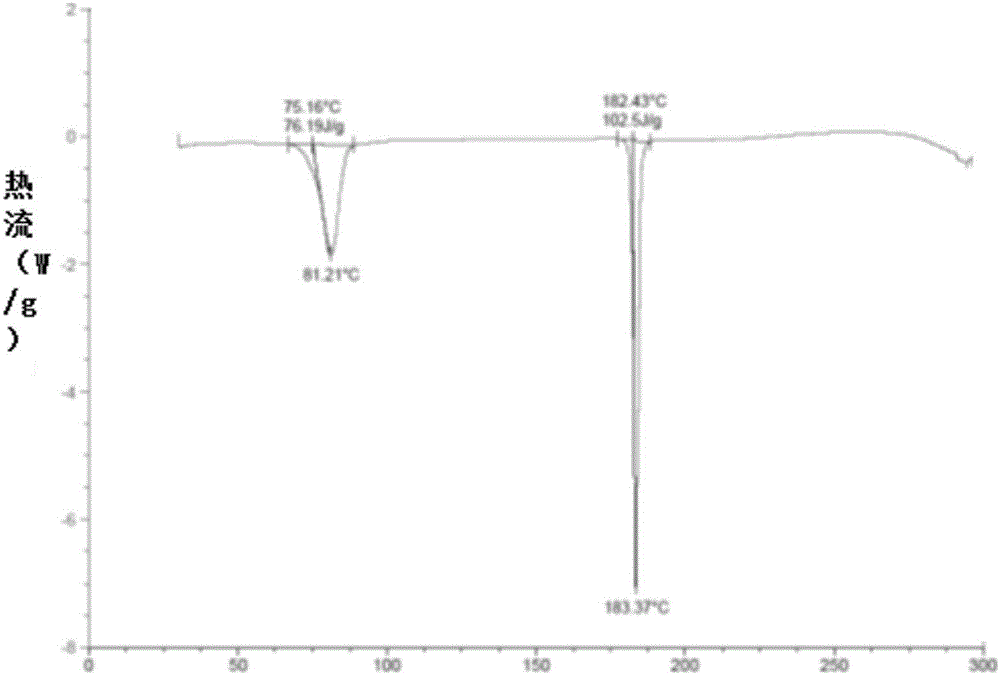
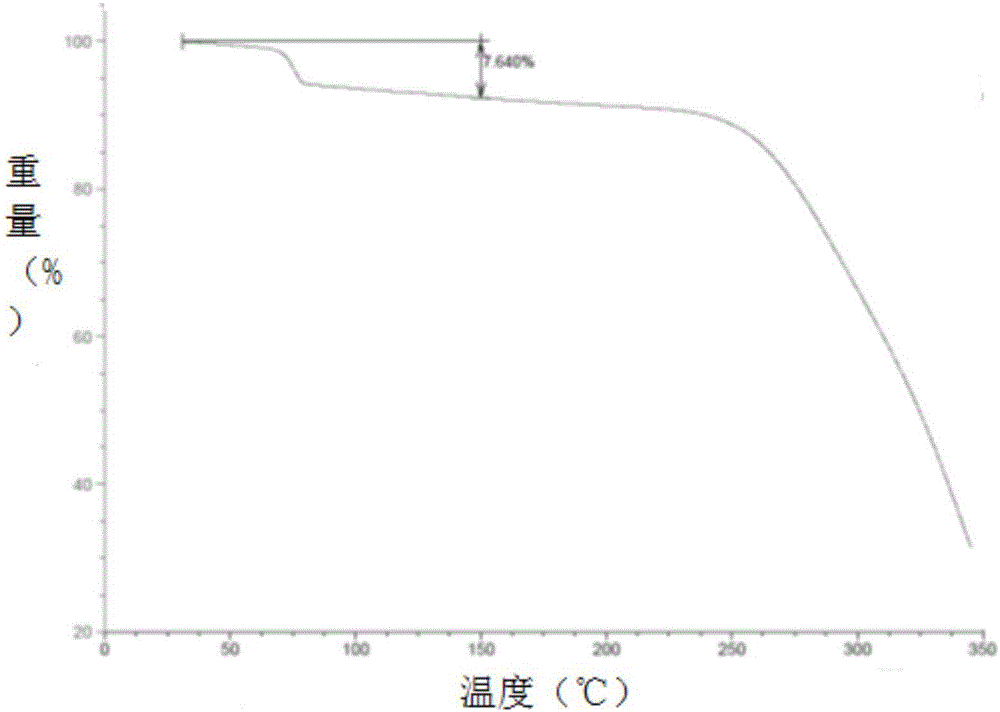
[0051] In a 50L reaction kettle, heat and dissolve 1.8kg of ebiprazole raw material (anhydrous substance) in 9L of dichloromethane and 1.8L of methanol, lower the temperature to 0-10°C, slowly add 18L of methanol dropwise, dropwise After completion, keep 0-10°C and stir at 120 rpm for 2 hours to crystallize, and filter to obtain a white solid filter cake. Vacuum drying (-0.08MPa--0.01MPa) at 45-55°C for 4-5 hours gave 1.68 kg of crystal form A of ebiprazole methanolate with a yield of 86.9% and a HPLC purity of 99.82%. Determination of its XRPD, DSC, DSC-TGA and infrared spectrum, its XRPD spectrum as figure 1 Shown; its DSC spectrum is shown in figure 2 shown; its DSC-TGA spectrum is as image 3 shown; its infrared spectrum is shown in Figure 4 Shown; The crystalline form A of the ebiprazole methanolate that embodiment 1 makes and reference preparation (otsukapharmaceutical.co., the ebiprazole anhydrous product that Otsuka Pharmaceutical produces) in 3% SDSpH6.8 Compari...
Embodiment 2
[0064] In a 500mL reaction bottle, heat and dissolve 18g of ebiprazole raw material (dihydrate) in 90mL of dichloromethane and 18mL of methanol, lower the temperature to 0-10°C, slowly add 180mL of methanol dropwise, after the dropwise addition , kept at 0-10° C. and stirred at a rate of 30 rpm for 2 hours to crystallize, and filtered to obtain a white solid filter cake. Vacuum (-0.08MPa--0.01MPa) drying at 45-55°C for 4-5 hours gave 16.1 g of crystal form A of ebiprazole methanolate, with a yield of 83.3% and a HPLC purity of 99.74%.
Embodiment 3
[0066] In a 500mL reaction bottle, heat and dissolve 18g of ebiprazole raw material (anhydrous substance) in 90mL of toluene and 18mL of methanol, lower the temperature to 0-10°C, slowly add 180mL of methanol dropwise, after the dropwise addition, Maintain 0-10°C and stir at 3000 rpm for 2 hours to crystallize, and filter to obtain a white solid filter cake. Vacuum drying (-0.08MPa--0.01MPa) at 45-55°C for 4-5 hours gave 16.5g of crystal form A of ebiprazole methanolate, with a yield of 85.3% and an HPLC purity of 99.89%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com