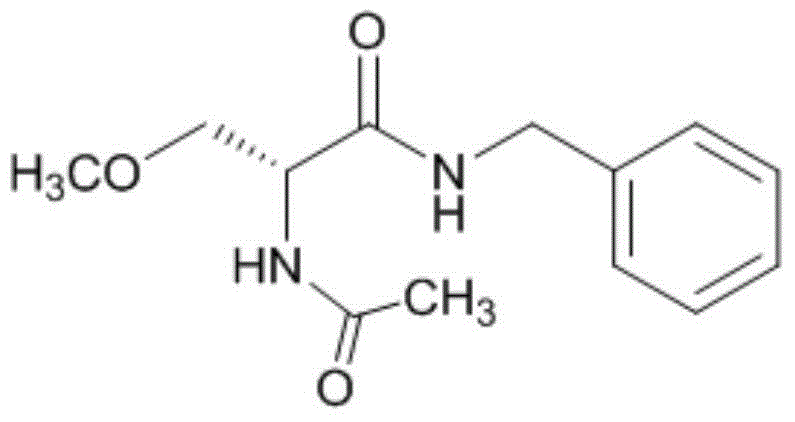
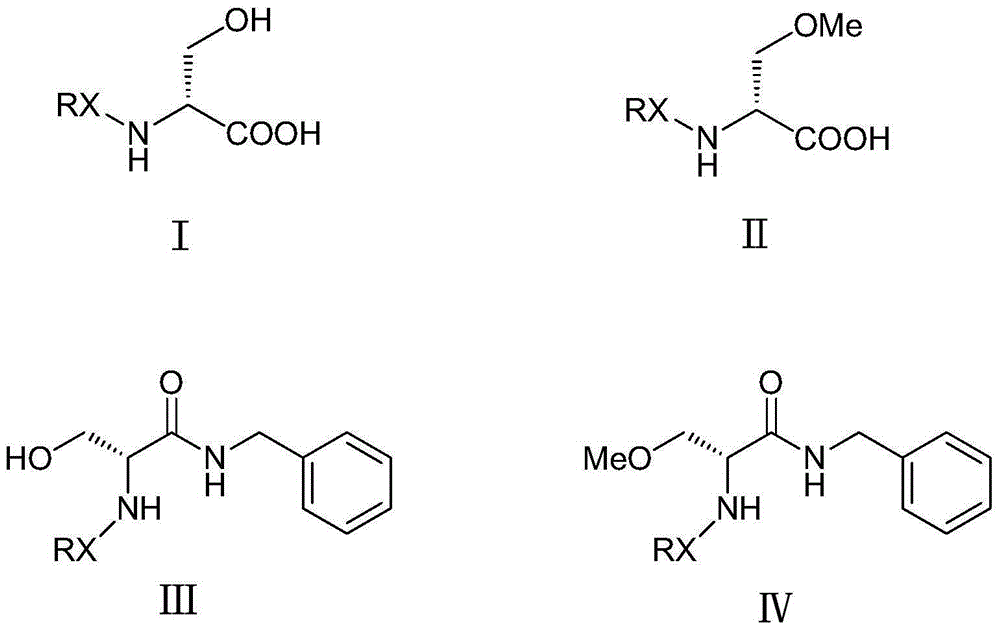
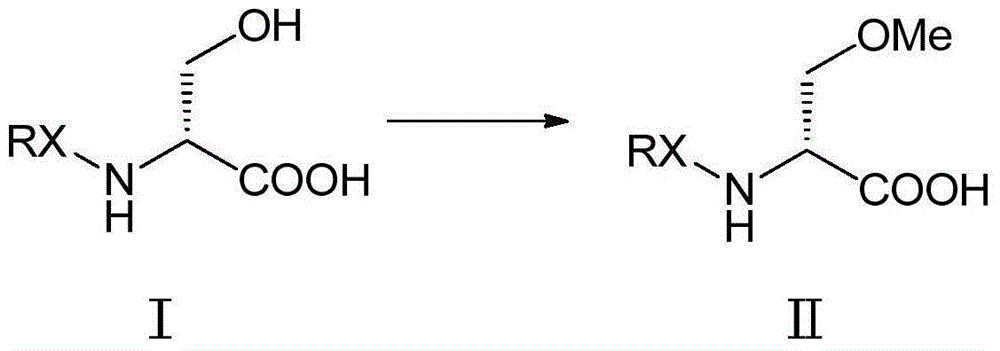
Methylation method of lacosamide intermediate
A technology of lacosamide and methylation, applied in chemical instruments and methods, organic chemistry, preparation of organic compounds, etc., to achieve the effect of increasing yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Into a 1000mL three-necked flask, add toluene (400mL) and N-tert-butoxycarbonyl-D-serine (Boc-D-serine) (40g, 0.195mol) (Intermediate I), and the stirring temperature is lowered to 0-5°C, Add dropwise 30% potassium hydroxide (36.5g, 0.195mol), stir for 30 minutes, then add methyl p-toluenesulfonate (72.65g, 0.390mol), tetrabutylammonium bromide (4g, 0.012mol), and then drop A 50% aqueous solution of potassium hydroxide (43.68 g, 0.390 mol) was added. After reacting at 0-5°C for 8 hours, (HPLC detects that the reaction of the raw materials is complete), add 200mL of water and separate the layers, extract the water layer with 100mL of toluene, discard the organic layer, cool the water layer to below 15°C and acidify it with 50% phosphoric acid to pH = 2-3.5, extracted with dichloromethane (200 mL / time * 3 times), combined organic layers, added anhydrous sodium sulfate (30 g) and stirred overnight. Filter and concentrate to dryness under reduced pressure. Obtained 42.9 g...
Embodiment 2
[0042] A solution of N-Boc-D-serine (40 g, 0.195 mol) in anhydrous THF (700 mL) was cooled to -10°C or below under a nitrogen atmosphere. 15% w / w n-butyllithium / hexane (268 mL, 0.432 mol) was added thereto through a dried dropping funnel while maintaining the temperature at 5°C or below. The resulting turbidity was reacted at 0-5°C for 1 hour. Methyl p-toluenesulfonate (58.03 g, 0.312 mol) was added while maintaining a temperature of 0-5°C, and the reaction mixture was stirred at 0-5°C for 12 hours. The reaction was quenched by the addition of water (220 mL), and the tetrahydrofuran and hexanes were evaporated under vacuum. The residue was washed with toluene (200 mL), then cooled to below 15°C and acidified with 50% phosphoric acid to pH=2-3.5, extracted with dichloromethane (200 mL / time*3 times), combined the organic layers and added anhydrous sodium sulfate ( 25 g) was stirred overnight. Filter and concentrate to dryness under reduced pressure. Obtained 41.8 g of light ...
Embodiment 3
[0044] Add toluene (820mL), intermediate III (50g), methyl p-toluenesulfonate (63.5g) and tetrabutylammonium bromide (5.4g) successively into the reaction flask, stir and cool to -1~2°C. Aqueous potassium hydroxide solution (40g / 34mL) was added at -1-2°C, and the addition was completed in about 5 minutes. After the dropwise addition, continue to control the temperature and react at -1 to 2°C for 4 to 4.5 hours (TLC detection or HPLC central control) to terminate the reaction. After the reaction was complete, water (400 mL) was added to separate the layers. The organic layer was washed successively with 5% phosphoric acid, saturated sodium bicarbonate, and 200 mL each of water. The solvent was distilled off under reduced pressure at 40-45°C to obtain an oil, which was directly used in the next reaction. HPLC purity 95%, chiral purity 99.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com