Pharmaceutical compositions and applications thereof, and pharmaceutical preparation
A technology of pharmaceutical preparations and compositions, applied in the fields of compositions and their uses, and pharmaceutical preparations, which can solve problems such as severe liver cirrhosis and multiple side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
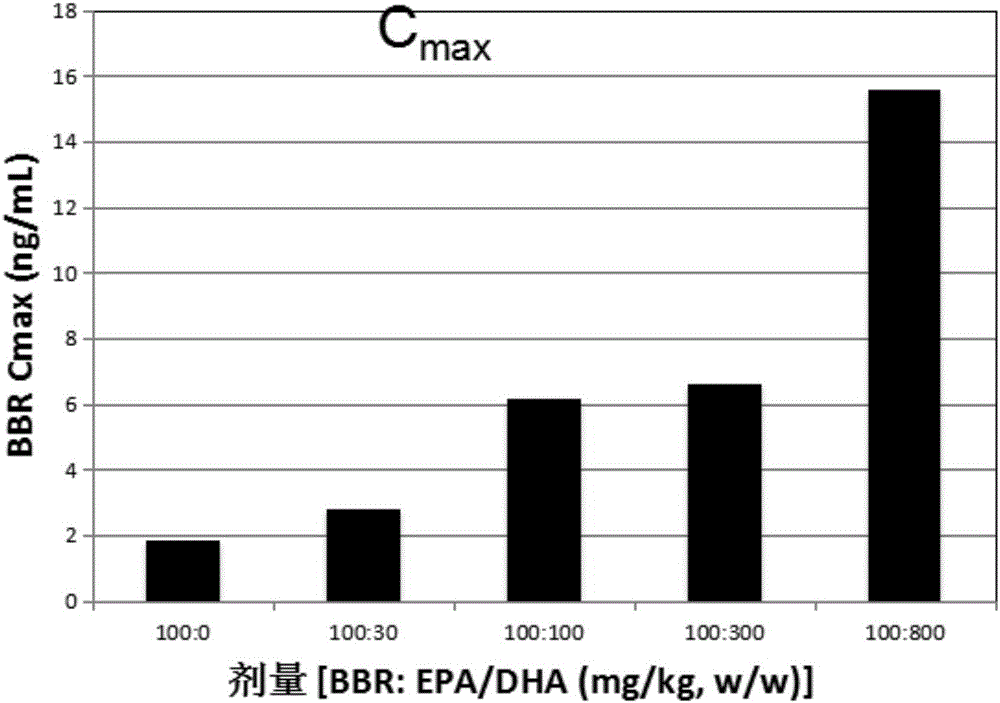
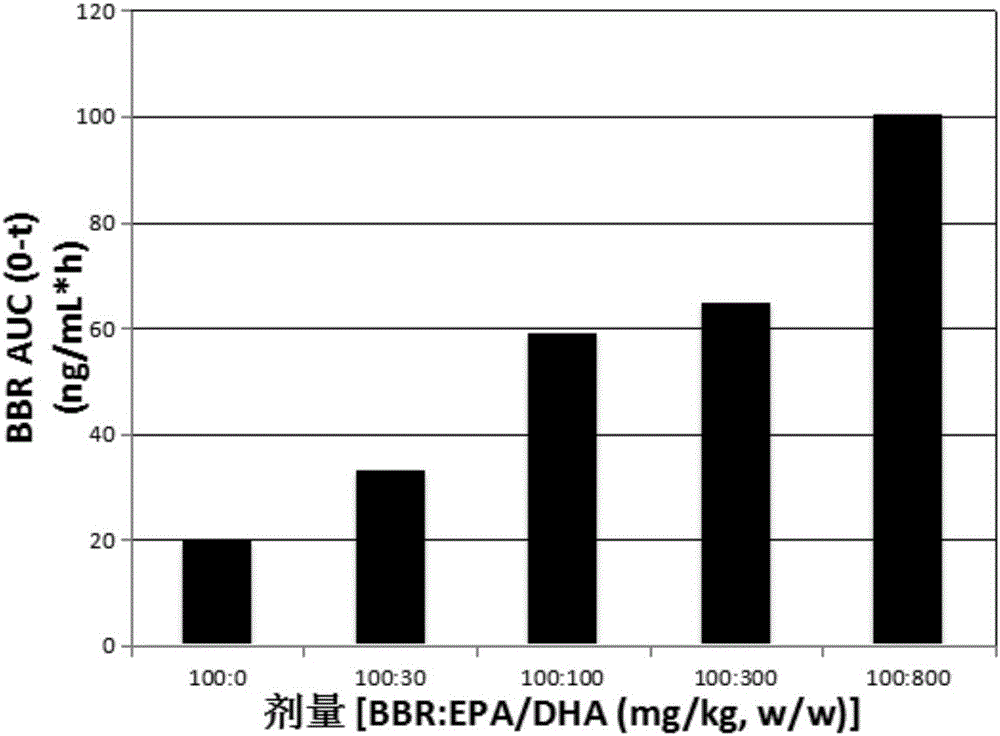
[0115] Example 1: Pharmacokinetics of berberine (BBR) and EPA / DHA formulations in rats and dogs learning situation
[0116] This example describes the pharmacokinetics of BBR and EPA and / or DHA formulations in rats and dogs.
[0117] Male SD rats, weighing 210-250g, were randomly divided into the following 3 groups after 7 days of adaptive feeding, with 3 rats in each group.
[0118]
[0119] Rats in each group were given the corresponding test substance suspended in 0.5% tragacanth gum by intragastric administration once. Blood samples (about 400 μL) were collected before administration and 15min, 30min, 1h, 1.5h, 2h, 4h, 8h and 24h after administration, placed in an anticoagulant tube containing sodium heparin, and centrifuged at 8000rpm, 4°C for 6 Plasma was separated within minutes, and samples were stored at -80°C until analysis.
[0120] The plasma concentration of BBR was determined and analyzed by tandem mass spectrometry (LC-MS / MS). use Professional5.2 (Ph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com