Medicinal composition for treating Alzheimer's disease and preparation method and application of medicinal composition
A composition and drug technology, applied in the field of pharmaceutical compositions for treating Alzheimer's disease and their preparation, can solve the problems of improving AD symptoms, limited improvement of clinical symptoms, unsatisfactory long-term efficacy and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] The preparation of embodiment 1 pharmaceutical composition
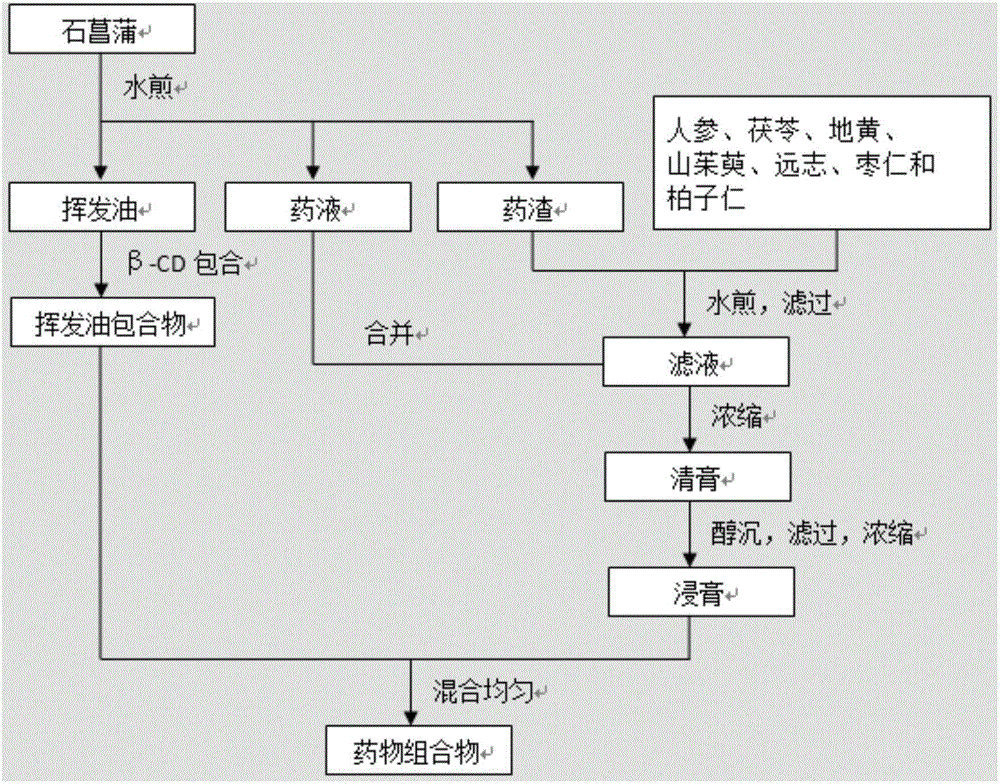
[0068] Add 8 times the amount of water to Acorus calamus (150g), extract for 10 hours, collect volatile oil (the extraction rate of volatile oil is 1.26%), and collect the remaining liquid medicine and dregs after extracting the volatile oil in separate devices. Add the volatile oil to the saturated aqueous solution of β-CD (calculated by weight ratio, W 挥发油 :W β-CD =1:8), stirred at 50°C for 2 hours, placed at low temperature for 24 hours, filtered, and dried the obtained filter cake at 40°C to obtain the clathrate of volatile oil of Acorus calamus. Take the dregs after extracting the volatile oil and mix them with ginseng stems and leaves (290g), Poria cocos (430g), Rehmannia glutinosa (860g), Cornus officinalis (860g), Zhiyuanzhi (150g), fried jujube kernels (430g) and Baiziren (430g) Add 18 times of water and decoct at 60°C for 3 times, each time for 1 hour, filter, combine the filtrate, combine the filt...
Embodiment 2
[0069] The preparation of embodiment 2 granules
[0070] Add excipients (1:1) to the pharmaceutical composition prepared in Example 1 to make granules. (as ginsenoside Rg 1 and ginsenoside Re content are quality inspection indicators, and the active ingredient content of the unit preparation is 0.85%)
Embodiment 3
[0071] The preparation of embodiment 3 pills
[0072] Add appropriate amount of sodium carboxymethyl starch and microcrystalline cellulose to the pharmaceutical composition prepared in Example 1 to adjust the total amount to 1000 g, wherein 577 grams of the pharmaceutical composition, mix well, add an appropriate amount of 70% ethanol as a wetting agent, mix Uniform, pelletized, dried (below 40°C), subpackaged to obtain. (as ginsenoside Rg 1 and ginsenoside Re content are the investigation indexes, and the active ingredient content of the unit preparation is about 0.97%)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com