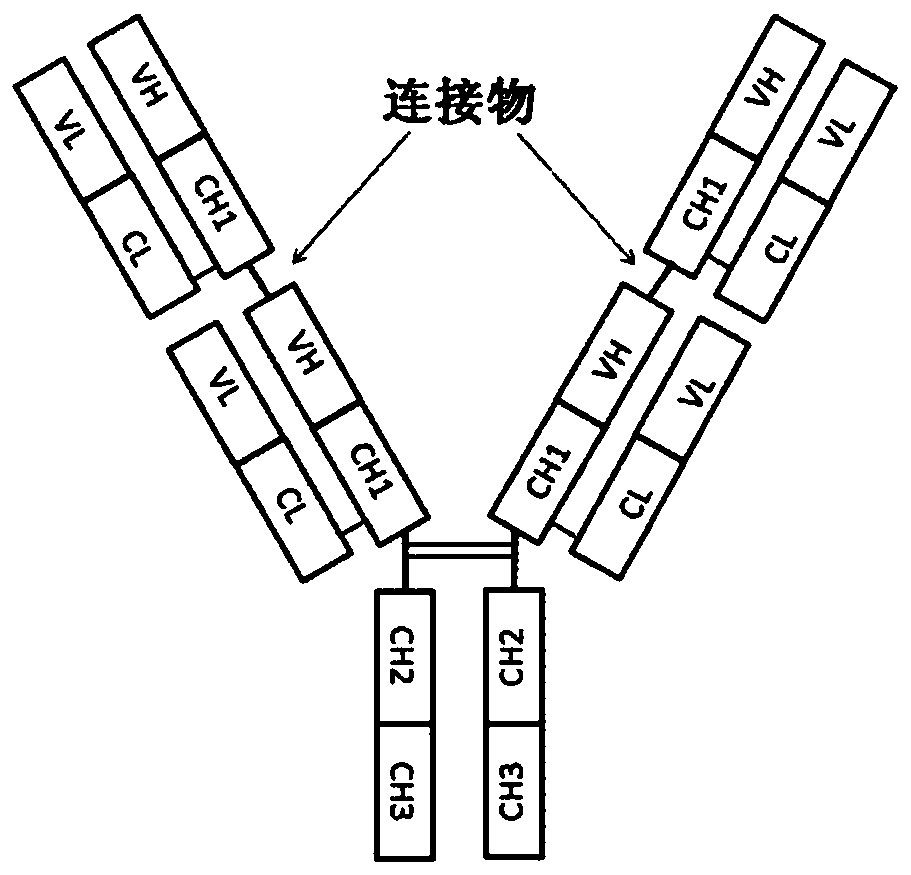
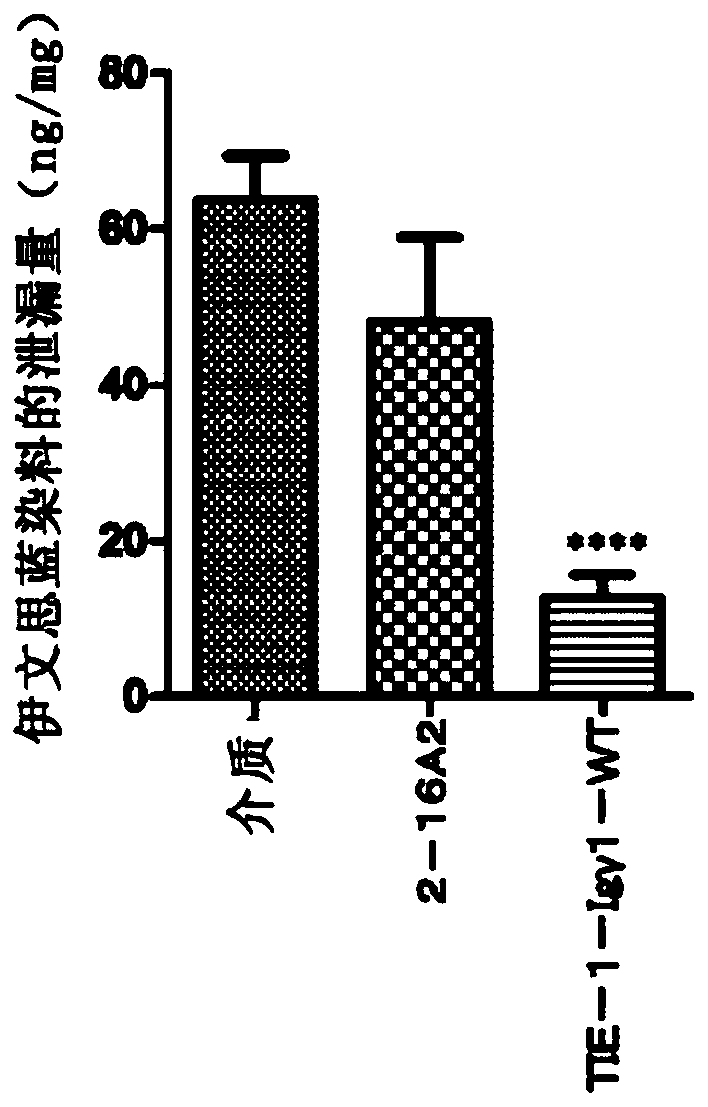
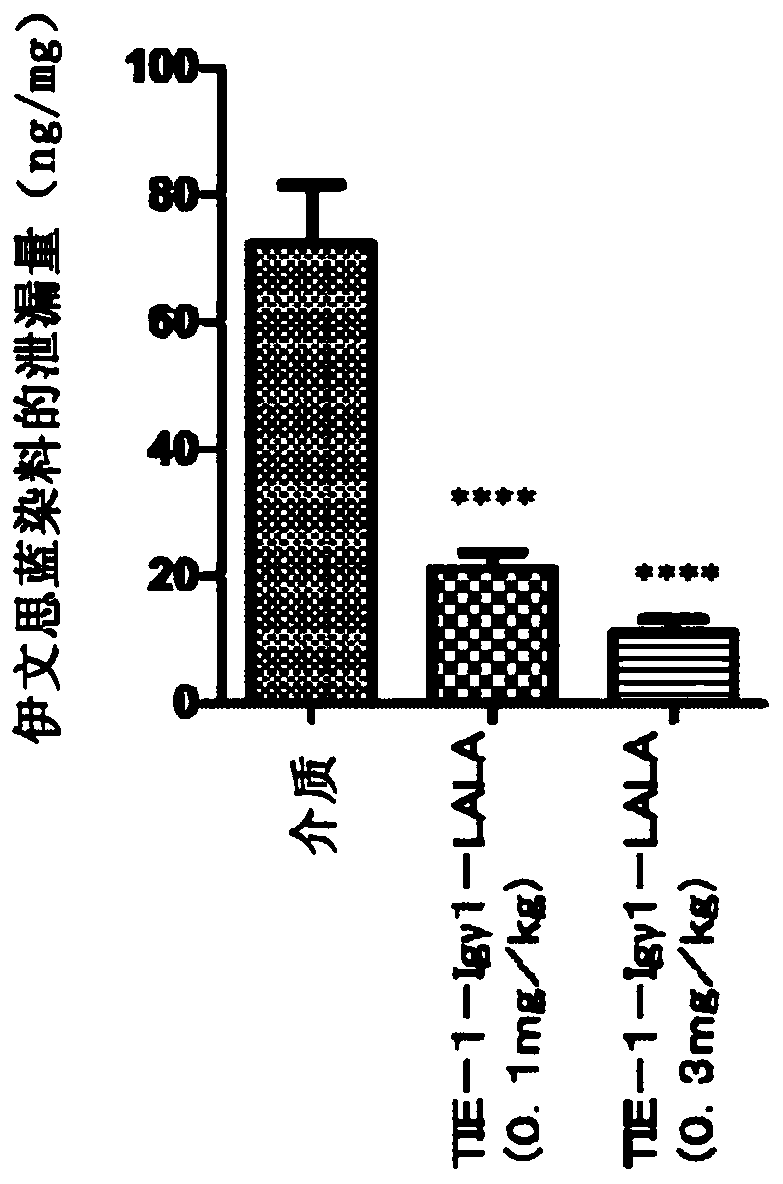
Novel anti-human tie-2 antibody
An antibody and antigen technology, applied in the direction of antibodies, antibody medical components, antibody mimics/scaffolds, etc., can solve problems such as increased retinal cell damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0246] (Example 1: Preparation of anti-human Tie2 antibody-producing hybridomas)
[0247] Antibodies were prepared using "VelocImmune" (VelocImmune Antibody Technology: Regeneron Corporation. (US Patent No. 6596541)) - Human Monoclonal Antibody Development Technology - Mouse. Recombinant human Tie2-Fc chimeric protein (R&D, 313-TI-100) was injected into VelocImmune mice for immunization with adjuvant for eliciting an immune response. According to a usual method, the lymph nodes of the immunized mice were extracted, and lymphocytes were collected and fused with mouse-derived myeloma cells SP2 / 0 (ATCC: CRL-1581) to prepare hybridomas. The hybridomas were monocloned, and each clone was cultured in CD Hybridoma Medium (Invitrogen) as a serum-free medium. Antibodies were purified from the resulting culture supernatant using a protein G column (GE Healthcare). The antibody obtained using the VelocImmune technology is an antibody having the variable region of a human antibody and t...
Embodiment 2
[0248] (Example 2: Cell ELISA assay)
[0249] To measure the antigen-binding activity of the antibodies, the CHO cells expressing human Tie2, CHO cells expressing monkey Tie2, CHO cells expressing rat Tie2, and CHO cells expressing mouse Tie2 were each evaluated by a cellular ELISA assay Binding of antibodies to human Tie2, monkey Tie2, rat Tie2 and mouse Tie2.
Embodiment 3
[0250] (Example 3: Evaluation of Competitive Activity Using Modified Ang-1)
[0251] To assess the Ang-2-competitive activity of the antibody, modified Ang-1 (Proc. Natl. Acad. Sci., 2004, Vol. 101, pp. 5547-5552, also known as COMP-Ang1) was evaluated with Inhibition of Tie2 binding. COMP-Ang1 is a modified Ang-1 in which a site not involved in binding to Tie2 is modified, and the binding ability to Tie2 from COMP-Ang1 is maintained (Proc.Natl.Acad.Sci.2004, Vol.101 , pp.5547-5552), and that Ang-1 and Ang-2 bind to the same site of Tie2 with the same level of affinity (Science, 1997, Vol.277, pp.55-60), it antagonizes The competitive role of Ang-2 can be assessed by evaluating the competitive role against COMP-Ang1.
[0252] The expression vector of COMP-Ang1 was introduced into HEK293 cells. COMP-Ang1 was purified from the culture supernatant of the HEK293 cells and biotinylated. The biotin-labeled COMP-Ang1 was mixed with the purified antibody obtained in Example 1, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com