A kind of a-π-d-π-a type bodipy derivative based on ethynyl bridging and preparation method thereof
A technology of ethynyl derivatives, which is applied in the field of boron fluoride complexed dipyrromethene derivatives, can solve the problems of limited species, complex synthesis, lack of adequate molecular design and synthetic route optimization, etc., and achieve low synthesis cost, π-π The accumulation phenomenon is obvious and the effect is easy to control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] Synthesis of target molecule BDP1:
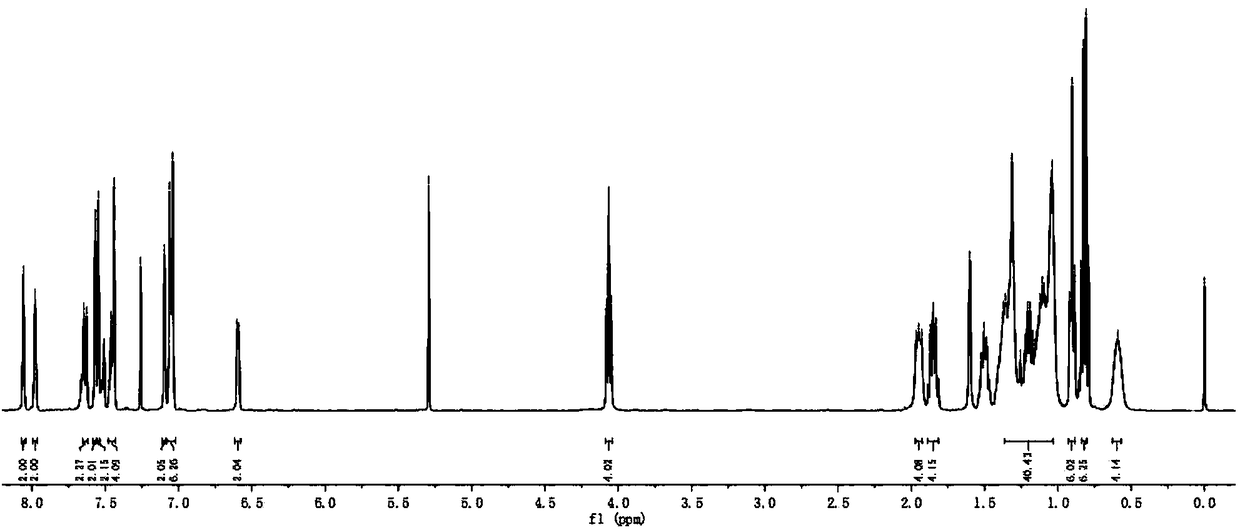
[0091] Add intermediate 3 (52mg, 0.1mmol), intermediate 4 (110mg, 0.25mmol), CuI (2.0mg, 0.01mmol), PdCl 2 (PPh 3 ) 2 (7mg, 0.01mmol), toluene (10mL), triethylamine (10mL), vacuumize, pass through argon protection, and react with magnetic stirring at room temperature for 12h. Stop the reaction, extract with ethyl acetate, wash with saturated brine, and dry over anhydrous magnesium sulfate. Filtered, and the filtrate was rotovapped to remove the solvent. The crude product was purified by silica gel (200-300 mesh) column chromatography [eluent, V (petroleum ether): V (ethyl acetate) = 5:1] to obtain purple-black solid compound BDP1 (88 mg), yield 72% . 1 H NMR (400MHz, CDCl 3 )δ: 8.06(s,2H),7.98(s,2H),7.64(d,J=7.6Hz,2H),7.57(s,2H),7.55(s,2H),7.48–7.43(m,4H ),7.10(s,2H),7.05(d,J=8.6Hz,6H),6.62–6.58(m,2H),4.06(t,J=6.5Hz,4H),1.97–1.93(m,4H) ,1.89–1.82(m,4H),1.37–1.03(m,40H),0.90(t,J=6.8Hz,6H),0.82(d,J=6.8Hz,6H),0.59(s,4H). 13 C N...
Embodiment 2
[0093] Synthesis of target molecule BDP2
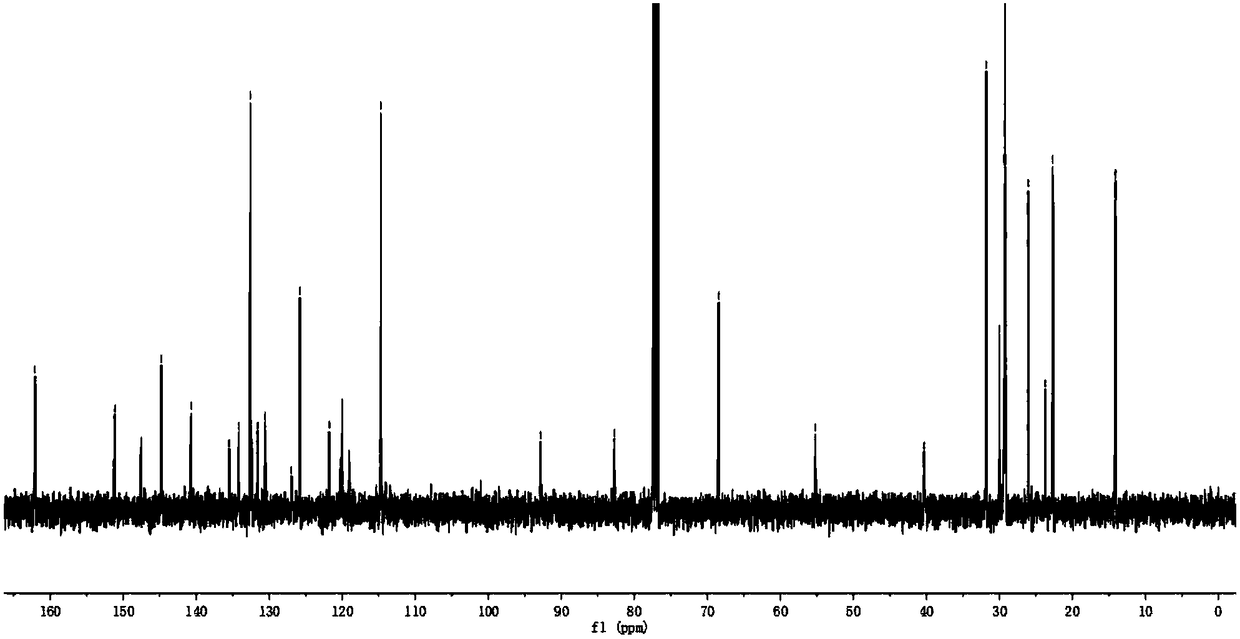
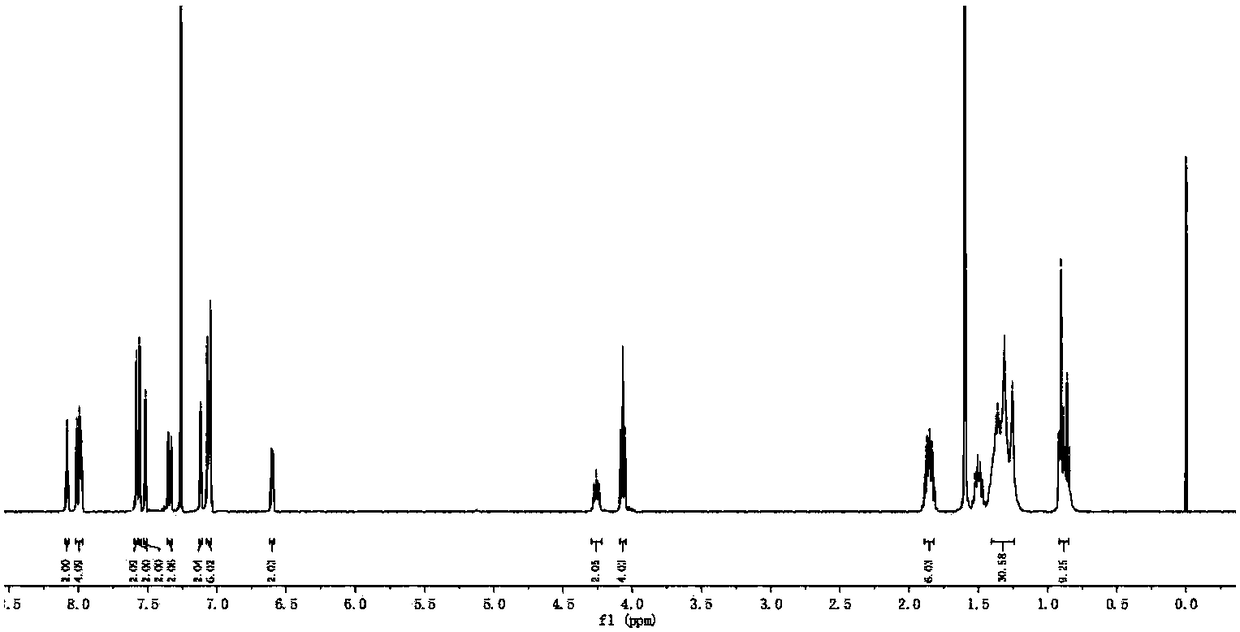
[0094] The synthesis method of BDP2 is similar to the synthesis method of BDP1. Intermediate 3 (52 mg, 0.1 mmol) and intermediate 5 (82 mg, 0.25 mmol) were used as substrates to purify the purple-black solid compound BDP2 (76 mg) with a yield of 68%. . 1 H NMR (400MHz, CDCl 3 )δ:8.08(s,2H),8.02–7.97(m,4H),7.58(d,J=1.9Hz,2H),7.57–7.55(m,2H),7.52(s,2H),7.34(dd ,J=8.1,1.2Hz,2H),7.12(s,2H),7.08–7.04(m,6H),6.60(dd,J=4.3,1.8Hz,2H),4.26(t,J=7.3Hz, 2H), 4.07(t, J=6.5Hz, 4H), 1.86(dd, J=14.2, 7.2Hz, 6H), 1.41–1.24(m, 30H), 0.92–0.85(m, 9H). 13 C NMR (101MHz, CDCl 3 )δ: 162.08, 147.53, 144.88, 144.72, 140.72, 135.48, 134.19, 132.59, 132.34, 131.59(s), 125.83(s), 122.72, 122.58 120.52(s), 120.30(s), 119.15 (s), 114.20(s), 111.85(s), 93.22(s), 82.29(s), 68.45(s), 43.30(s), 31.84(s), 29.39, 29.36, 29.27, 29.21, 29.16, 28.97 , 27.31(s), 26.06(s), 22.69, 22.63, 14.14, 14.10. MALDI-TOF-MS, m / z: calcd for C 70 h 75 B 2 f 4 N 5 o 2 [M] + :...
Embodiment 3
[0096] Synthesis of target molecule BDP3
[0097] The synthesis method of BDP3 is similar to the synthesis method of BDP1. Intermediate 3 (52mg, 0.1mmol) and intermediate 6 (124mg, 0.25mmol) were used as substrates to obtain purplish-black solid compound BDP3 (74mg) with a yield of 58%. . 1 H NMR (400MHz, CDCl 3 )δ:8.04(s,2H),8.00(s,2H),7.56(d,J=7.1Hz,6H),7.07(dd,J=10.8,7.9Hz,8H),6.61(d,J=2.5 Hz, 2H), 4.24(t, J=6.5Hz, 4H), 4.07(t, J=6.5Hz, 4H), 1.85(dd, J=13.3, 6.6Hz, 8H), 1.33(d, J=18.3 Hz,40H),0.92–0.88(m,12H). 13 C NMR (101MHz, CDCl 3 )δ:162.19,147.87,145.62,145.36,144.36,143.83,135.68,134.09,132.83,132.61,131.94,131.54,130.34,125.70,125.11,122.89,119.38,114.77,88.67,85.46,74.17,68.47,53.43,31.85 ,31.83,30.52,29.71,29.41,29.35,29.26,29.15,26.04,26.03,22.68,14.13.MALDI-TOF-MS,m / z: calcd for C 76 h 88 B 2 f 4 N 4 o 4 S 2 [M]+ :1282.637; found1282.170.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com