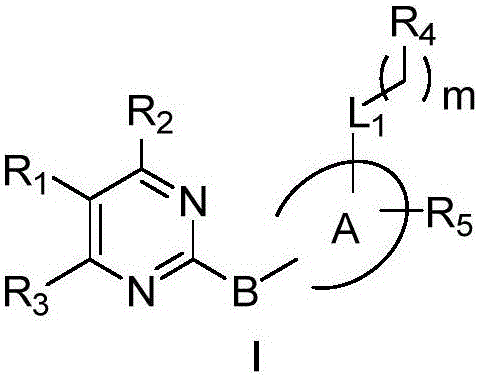
2-polysubstituted aromatic ring-pyrimidine derivative and preparation and medical application
A technology of derivatives and pyrimidines, used in pharmaceutical formulations, drug combinations, anti-tumor drugs, etc., can solve problems such as poor selectivity, toxic and side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0152] Preparation Example 1.N 4 -Methyl-5-(1-methyl-1H-pyrazol-4-yl)-N 2 -(1-methyl-5-(piperidin-4-oxyl)pyrazol-3-yl)-2,4-diaminopyrimidine (compound 1)
[0153]
[0154] Step 1. Synthesis of N-tert-butoxycarbonyl-4-((3-bromo-1-methyl-1H-pyrazol-5-yl)oxy)piperidine (Intermediate 1-2)
[0155]
[0156]Dissolve N-tert-butoxycarbonyl-4-hydroxypiperidine (460mg, 2.29mmol) in anhydrous THF (7.2mL), and add 60% sodium hydride (108mg, 4.5mmol) in batches under ice-cooling. Stir for 10 min. Raise the temperature to 35°C and stir for 10 minutes. 3-Bromo-1-methyl-5-nitropyrazole (360mg, 1.75mmol) was placed in a 50mL three-necked flask, and anhydrous THF (4.8mL) was added under nitrogen protection, and the sodium salt prepared above was slowly Add it dropwise to the reaction solution (the dropwise addition is completed in about 20 minutes), and stir at room temperature for 1 hour. The reaction was quenched by adding saturated ammonium chloride solution, and the solvent was r...
preparation Embodiment 2
[0169] Preparation Example 2.N 4 -Methyl-5-(1-methyl-1H-pyrazol-4-yl)-N 2 -(3-(piperidin-4-oxyl)isoxazol-5-yl)-2,4-diaminopyrimidine (compound 2)
[0170]
[0171] Step 1. Synthesis of N-tert-butoxycarbonyl-4-((5-bromo-isoxazol-3-yl)oxy)piperidine (Intermediate 1-8)
[0172]
[0173] Synthetic steps Reference Example 1 Step 1. Compound 1-8 was prepared from 5-bromo-3-nitropyrazole (compound 1-7) by a synthetic method similar to compound 1-2. Yield: 63%; LCMS: m / z=348[M+1] + .
[0174] Step 2.N 4 -Methyl-5-(1-methyl-1H-pyrazol-4-yl)-N 2 Synthesis of -(3-(piperidin-4-oxyl)isoxazol-5-yl)-2,4-diaminopyrimidine (Compound 2)
[0175]
[0176] For the synthesis steps, refer to step 5 of Example 1. Using a synthesis method similar to compound 1, using intermediates 1-8 and 1-6 as raw materials, compound 2 was prepared. Yield: 65%; LCMS: m / z=371[M+1] + .
preparation Embodiment 3
[0177] Preparation Example 3.N 4 -Methyl-5-(1-methyl-1H-pyrazol-4-yl)-N 2 -(1-methyl-2-(piperidin-4-oxyl)imidazol-4-yl)-2,4-diaminopyrimidine (Compound 3)
[0178]
[0179] Step 1. Synthesis of N-tert-butoxycarbonyl-4-((4-bromo-1-methyl-1H-imidazol-2-yl)oxy)piperidine (Intermediate 1-10)
[0180]
[0181] Synthesis steps Reference Example 1 Step 1. Compound 1-10 was prepared from 4-bromo-1-methyl-2-nitroimidazole (compound 1-9) by a method similar to that of compound 1-2. Yield: 67%; LCMS: m / z=361[M+1] + .
[0182] Step 2.N 4 -Methyl-5-(1-methyl-1H-pyrazol-4-yl)-N 2 Synthesis of -(1-methyl-2-(piperidin-3-oxyl)imidazol-4-yl)-2,4-diaminopyrimidine (Compound 3)
[0183]
[0184] For the synthesis steps, refer to step 5 of Example 1. Compound 3 was prepared by using a synthesis method similar to compound 1, using intermediates 1-10 and 1-6 as raw materials. Yield: 65%; LCMS: m / z=384[M+1] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com