Benzalkonium chloride solution and preparation method thereof
A technology of benzalkonium chloride and solution, which is applied in the field of benzalkonium chloride solution and its preparation, can solve the problems of beautifying wounds and other problems, and achieve the effects of enhanced adsorption and penetration, enhanced bactericidal effect, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
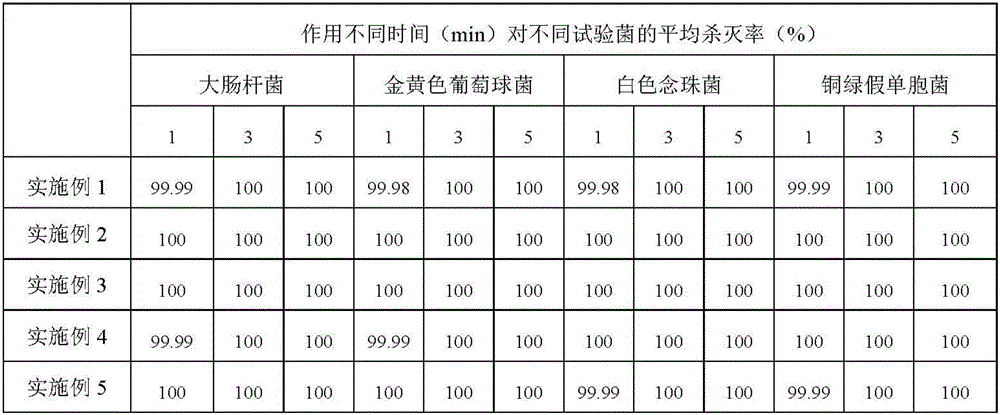
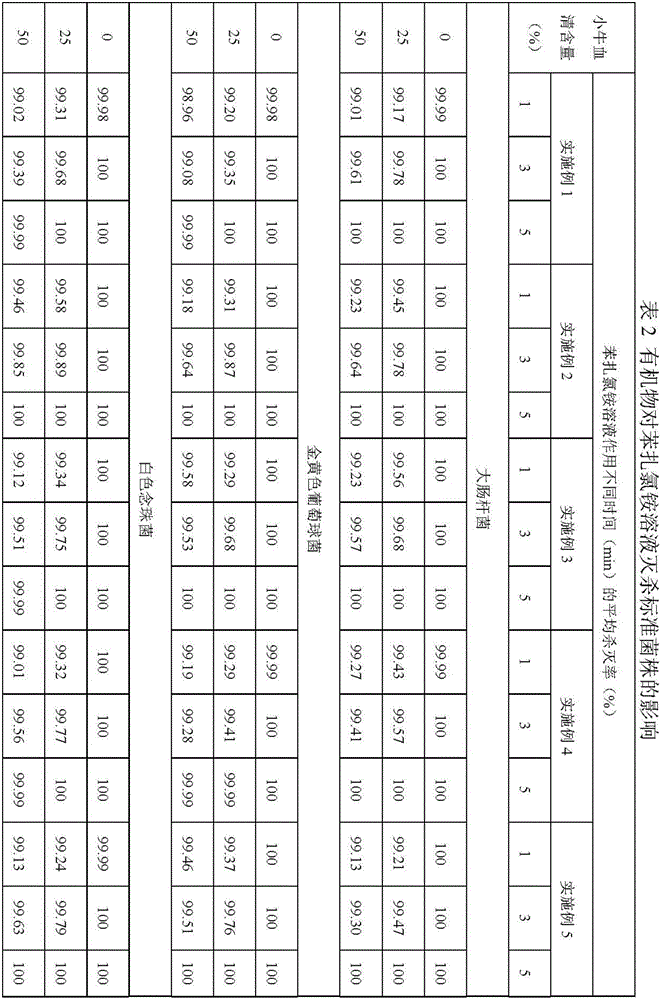
Examples
Embodiment 1
[0032] A benzalkonium chloride solution, which comprises the following components in mass percent concentration: 0.13% benzalkonium chloride, 0.05% triclosan, 1% ethoxylated fatty acid methyl ester, 0.5% EDTA-2Na, aloe extract 5% vitamin E, 1% vitamin E, 1% sodium hyaluronate, 1.5% citric acid, and 89.82% water for injection.
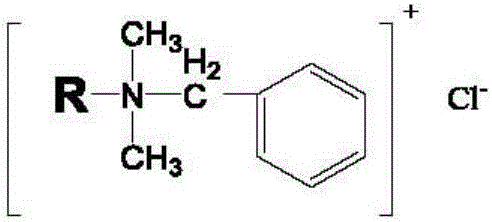
[0033] Among them, C in the structure of benzalkonium chloride 12 : C 14 : C 16 =40%:30%:5%.
[0034] Its preparation method is as follows:
[0035] (1) According to the requirement of mass percentage, take each component raw material;
[0036] (2) Add the same mass of water for injection into triclosan and ethoxylated fatty acid methyl ester, heat to 60°C, stir evenly, and cool to normal temperature after fully dissolving to prepare mixed solution A;
[0037] (3) Add benzalkonium chloride, EDTA-2Na, aloe extract, vitamin E, sodium hyaluronate and citric acid to equal quality water for injection in turn, heat to 40°C, stir evenly, and cool down aft...
Embodiment 2
[0040] A benzalkonium chloride solution, which comprises the following components in mass percent concentration: 0.21% benzalkonium chloride, 0.2% triclosan, 2% ethoxylated fatty acid methyl ester, 2% EDTA-2Na, aloe extract 10% vitamin E, 5% vitamin E, 5% sodium hyaluronate, 3% malic acid, and 72.59% water for injection.
[0041] Among them, C in the structure of benzalkonium chloride 12 : C 14 : C 16 =50%:40%:10%.
[0042] Its preparation method is as follows:
[0043] (1) According to the requirement of mass percentage, take each component raw material;
[0044](2) Add the same mass of water for injection into triclosan and ethoxylated fatty acid methyl ester, heat to 80°C, stir evenly, and cool to normal temperature after fully dissolving to prepare mixed solution A;
[0045] (3) Add benzalkonium chloride, EDTA-2Na, aloe extract, vitamin E, sodium hyaluronate and malic acid to equal quality water for injection in turn, heat to 60°C, stir evenly, and cool down after ful...
Embodiment 3
[0048] A benzalkonium chloride solution, which comprises the following components in mass percent concentration: 0.17% benzalkonium chloride, 0.1% triclosan, 1.3% ethoxylated fatty acid methyl ester, 0.75% EDTA-2Na, aloe extract 8%, vitamin E 3%, sodium hyaluronate 3%, citric acid 2%, water for injection 81.68%.
[0049] Among them, C in the structure of benzalkonium chloride 12 : C 14 : C 16 =45%:35%:5%.
[0050] Its preparation method is as follows:
[0051] (1) According to the requirement of mass percentage, take each component raw material;
[0052] (2) Add the same mass of water for injection into triclosan and ethoxylated fatty acid methyl ester, heat to 70°C, stir evenly, and cool to normal temperature after fully dissolving to prepare mixed solution A;
[0053] (3) Add benzalkonium chloride, EDTA-2Na, aloe extract, vitamin E, sodium hyaluronate and citric acid to equal quality water for injection in turn, heat to 55°C, stir evenly, and cool down after fully disso...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com