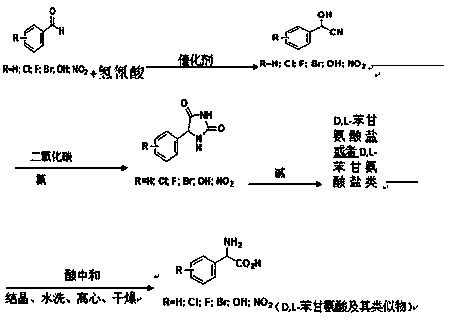
A kind of preparation method of d,l-phenylglycine and its analogs
A technology for phenylglycine and its analogues, which is applied in the field of preparation of D,L-phenylglycine and its analogues, which can solve the problems of complex preparation methods, many by-product inorganic salts, and low product yields, and achieve simple and feasible process flow , less by-product inorganic salts, high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Start the benzaldehyde (99.8% content) metering pump with a 600Kg / h drop rate and hydrocyanic acid (99% content) metering pump with a feed rate of 158.7Kg / h and add it to the 3000L reactor A. After feeding for 10 minutes, feed the reactor Continuously add saturated aqueous sodium bicarbonate solution to adjust the pH value at 5.0~5.5, keep the reaction temperature at 25°C, keep the liquid level of reactor A at 65%, and then overflow into the 5000L reactor B, and the reaction temperature in reactor B Keep at 30°C, analyze the residual amount of benzaldehyde by HPLC, when the residual amount of benzaldehyde is less than 500ppm, it is regarded as the end of the reaction, and the qualified cyanohydrin is extracted from the reactor B, and acidified with 85% phosphoric acid to pH 3 , The yield of cyanohydrin ≥99.9%.
[0031] The aqueous solution of carbon dioxide and ammonia (containing 13% carbon dioxide by mass percent and 8% ammonia by mass percent) and cyanohydrin are con...
Embodiment 2
[0035] Add benzaldehyde with a mass percentage of 99.5% into the absorption tower, then pass through the deaminated hydrocyanic acid mixed gas (hydrocyanic acid content is 10%) into the tower, and add sodium acetate aqueous solution to the tower at the same time Adjust the pH inside the tower to 5.5, control the temperature of absorbing hydrocyanic acid to 30°C-35°C, the exhaust gas absorbed by the tower is then cryogenically refluxed into the tower, and the tail gas that has not been liquefied directly enters the incineration system for incineration; central control The residual amount of benzaldehyde is analyzed by HPLC. When the residual amount of benzaldehyde is less than 500ppm, it is regarded as the end point of the reaction. The qualified cyanohydrin is extracted from the absorption tower, acidified with 70% sulfuric acid to pH 3, and the yield of cyanohydrin is ≥ 99.9%.
[0036] The aqueous solution of carbon dioxide and ammonia (containing 13% carbon dioxide by mass p...
Embodiment 3
[0040] Start benzaldehyde (content 99.8%) metering pump with 600Kg / h drip rate and hydrocyanic acid aqueous solution (content 60%) metering pump with 264.5Kg / h feeding speed and join in 3000L reactor A, feed 10 minutes later, to reaction Continuously add sodium citrate aqueous solution to the kettle to adjust the pH value at 5.0-5.5, keep the reaction temperature at 25°C, keep the liquid level of reactor A at 65%, and then overflow into the 5000L reactor B, and the reaction temperature in reactor B Keep at 30°C, analyze the residual amount of benzaldehyde by HPLC, when the residual amount of benzaldehyde is less than 500ppm, it is regarded as the end of the reaction, and the qualified cyanohydrin is extracted from the reactor B, and acidified with 85% phosphoric acid to pH 3 , The yield of cyanohydrin ≥99.9%.
[0041] The aqueous solution of carbon dioxide and ammonia (containing 13% carbon dioxide by mass percent and 8% ammonia by mass percent) and cyanohydrin are continuousl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com