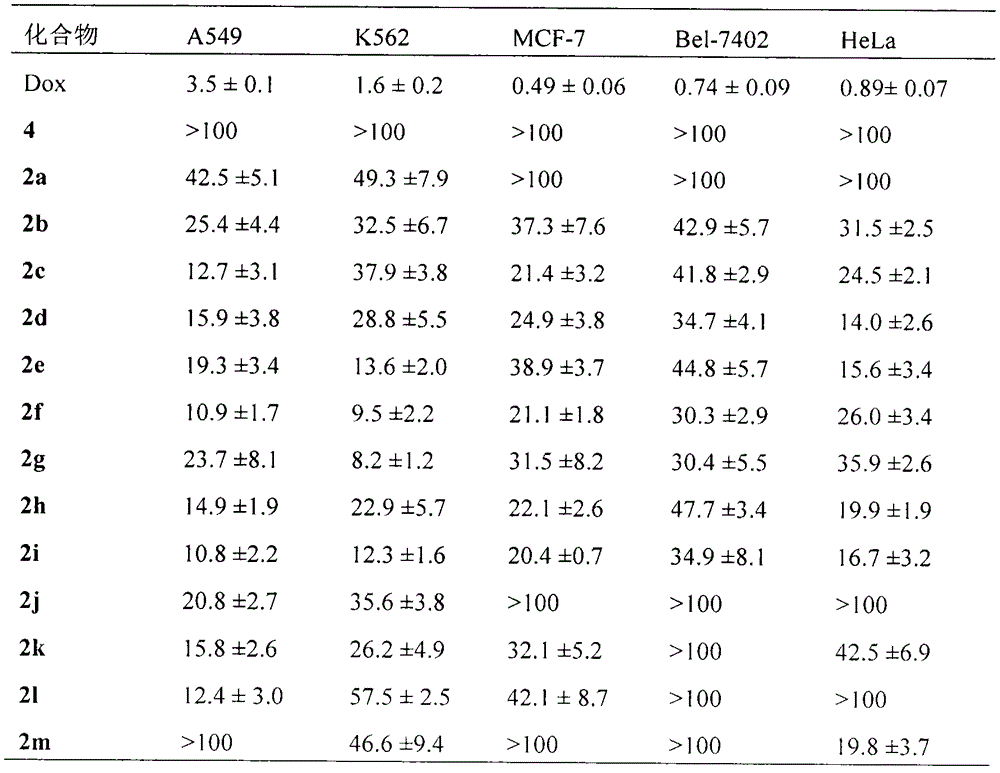
Novel indoles compound having anti-tumor metastasis activity and anti-inflammatory activity, as well as synthesis and application of novel indoles compound
A reactive, hydroxyethyl technology, applied in the fields of inhibiting tumor weight gain, anti-tumor lung metastases and anti-inflammatory activities in S180 tumor-bearing mice, which can solve problems such as low quality of life and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
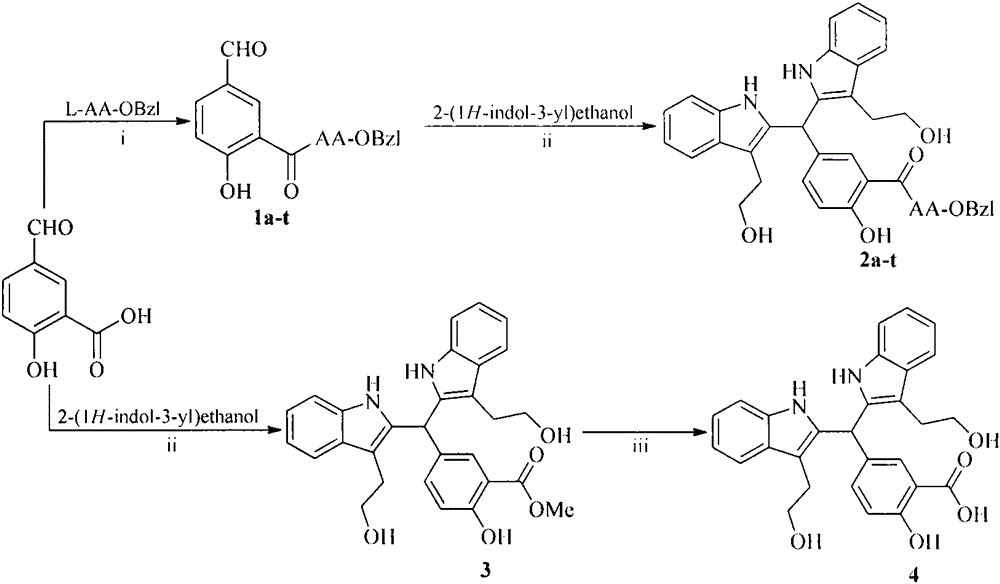
[0020] Embodiment 1 liquid phase connection peptide reaction general method
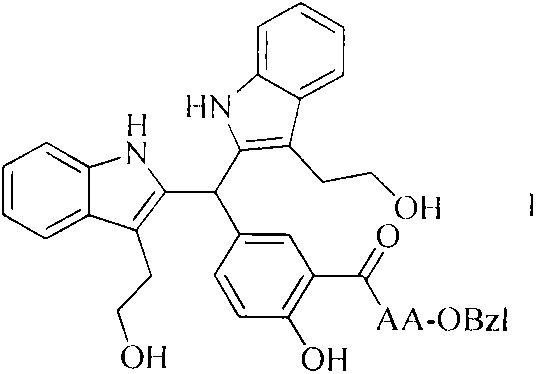
[0021] Dissolve the carboxyl-terminally exposed compound in anhydrous tetrahydrofuran (THF), add N-hydroxybenzotriazole (HOBt) to the obtained solution, and slowly add N,N-dicyclohexylcarbon dissolved in anhydrous THF under ice-cooling Diimine (DCC), stirred at 0°C for 15 minutes to obtain the reaction solution (I). The carboxy-terminally protected amino acid was also dissolved in anhydrous THF, adjusted to pH 9 with N-methylmorpholine (NMM), then mixed with the reaction solution (I), maintained at pH 9 with N-methylmorpholine, and stirred at room temperature for 10 h. The progress of the reaction was monitored by TLC. After the raw material point disappeared, the reaction mixture was filtered, and the filtrate was concentrated under reduced pressure to obtain a viscous substance, which was dissolved in ethyl acetate or dichloromethane, and the obtained solution was sequentially washed with 5% NaHCO...
Embodiment 2
[0022] Example 2 Preparation of 5-(bis(3-(2-hydroxyethyl)-1H-indole-2)methyl)-2-hydroxybenzoic acid methyl ester (3)
[0023] 1.66g (10mmol) of methyl 5-formylsalicylate, 3.22g (20mmol) of indole alcohol and 0.17g (1mmol) of p-toluenesulfonic acid were dissolved in 50mLTHF, reacted at 60°C for 10h, and monitored by TLC (CH 2 Cl 2 :CH 3 OH=30:1) the raw material point disappeared, the reaction was stopped, cooled to room temperature, concentrated to dryness under reduced pressure, the residue was dissolved in dichloromethane, and 3.8 g (78%) of light yellow powder was obtained by silica gel column chromatography. IR: 3375, 3055, 1672, 1487, 1456, 1208, 1088, 738cm -1 ;FT-MS(m / e):485.20627[M+H] + (calculated: 484.19982); 1 H NMR (800MHz, DMSO-d6) δ=10.52(s, 2H), 10.47(s, 1H), 7.58(s, 1H), 7.50(d, J=8.0Hz, 2H), 7.30(d, J= 8.0Hz, 2H), 7.25(d, J=8.8Hz, 2H), 7.04(t, J=7.2Hz, 2H), 6.97(t, J=7.2Hz, 2H), 6.95(d, J=8.8Hz , 1H), 6.08 (s, 1H), 3.80 (s, 3H), 3.53 (m, 2H), 3.49 (m, 2H...
Embodiment 3
[0024]Example 3 Preparation of 5-(bis(3-(2-hydroxyethyl)-1H-indole-2)methyl)-2-hydroxybenzoic acid (4)
[0025] Under ice bath, 1.2g (2.4mmol) 5-(bis(3-(2-hydroxyethyl)-1H-indole-2)methyl)-2-hydroxybenzoic acid methyl ester (3) was dissolved in methanol, added 4N NaOH aqueous solution, adjust the pH value to 12, react for 48h, TLC plate monitoring (CH 2 Cl 2 :CH 3 OH=20:1) The raw material point disappears and the reaction is complete. saturated KHSO 4 Adjust the reaction solution to neutral, concentrate under reduced pressure to remove methanol, continue to adjust the pH value of the residue to 3, extract 3 times with ethyl acetate, combine the ethyl acetate phases, dry with anhydrous sodium sulfate, filter, and concentrate under reduced pressure to obtain 1.08 g (93%) of the title compound as a light gray solid. IR: 3367, 1667, 1457, 1215, 1038, 740cm -1 ;FT-MS (m / e): 469.17775[M-H] - (calculated: 470.18417); 1 HNMR (800MHz, DMSO-d6) δ=10.53(s, 2H), 7.53(s, 1H), 7.50...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com