Separation and determination method of desonide and related impurities
A desonide and impurity technology, applied in the field of analytical chemistry, can solve the problems of poor separation of degradation impurities, poor durability, harsh conditions, etc., and achieve the effects of strong specificity, good applicability and accurate results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] The determination of embodiment 1 HPLC separation detection method of the present invention
[0052] Based on the limitations of using the USP38 version of desonide raw material drug quality standard reversed-phase liquid chromatography to determine the relevant impurities of desonide and its preparations, the inventors of the present application have repeatedly screened and finally determined the separation and determination of the reversed-phase HPLC method of the present invention. The method for neide and related impurities is specifically: prepare desonide need testing solution, adopt high-performance liquid chromatograph sample introduction analysis to measure the peak area of each material in desonide need test solution, normalize or The relative content of each impurity is calculated by self-contrast method; the chromatographic column that described reverse-phase HPLC method adopts is the chromatographic column of octadecylsilane bonded silica gel filler, and t...
Embodiment 2
[0064] The detection of embodiment 2 sensitivity
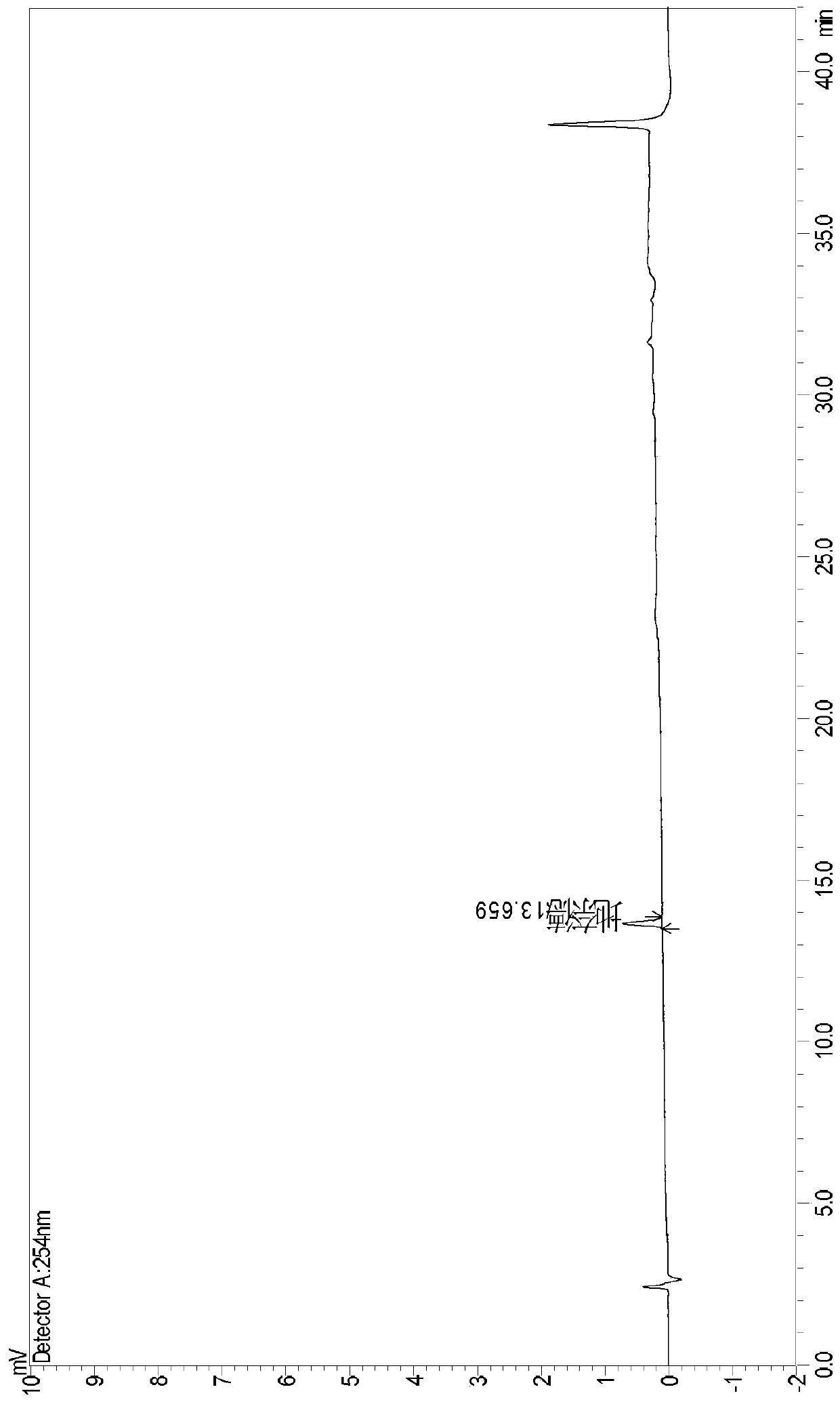
[0065] Weigh an appropriate amount of desonide reference substance, ultrasonically dissolve and dilute it with 0.1% phosphoric acid aqueous solution-acetonitrile (volume ratio 60:40) to make a solution containing about 0.25 μg desonide per 1 ml, as a sensitivity test solution.
[0066] Accurately measure 10 μ l of the above-mentioned sensitivity test solution and inject it into a liquid chromatograph, measure according to the best chromatographic conditions in Example 1, and record the chromatogram, such as figure 1 shown. Depend on figure 1 It can be seen that in the sensitivity chromatogram, the desonide peak (S / N) is greater than 10.0. In the figure, the ordinate is the peak area (unit: mV / mAU), the abscissa is the time (unit: min), and the following chromatograms have no special instructions, the ordinate is the peak area (unit: mV / mAU), and the abscissa is the peak area (unit: mV / mAU). The mark is time (unit: min).
Embodiment 3
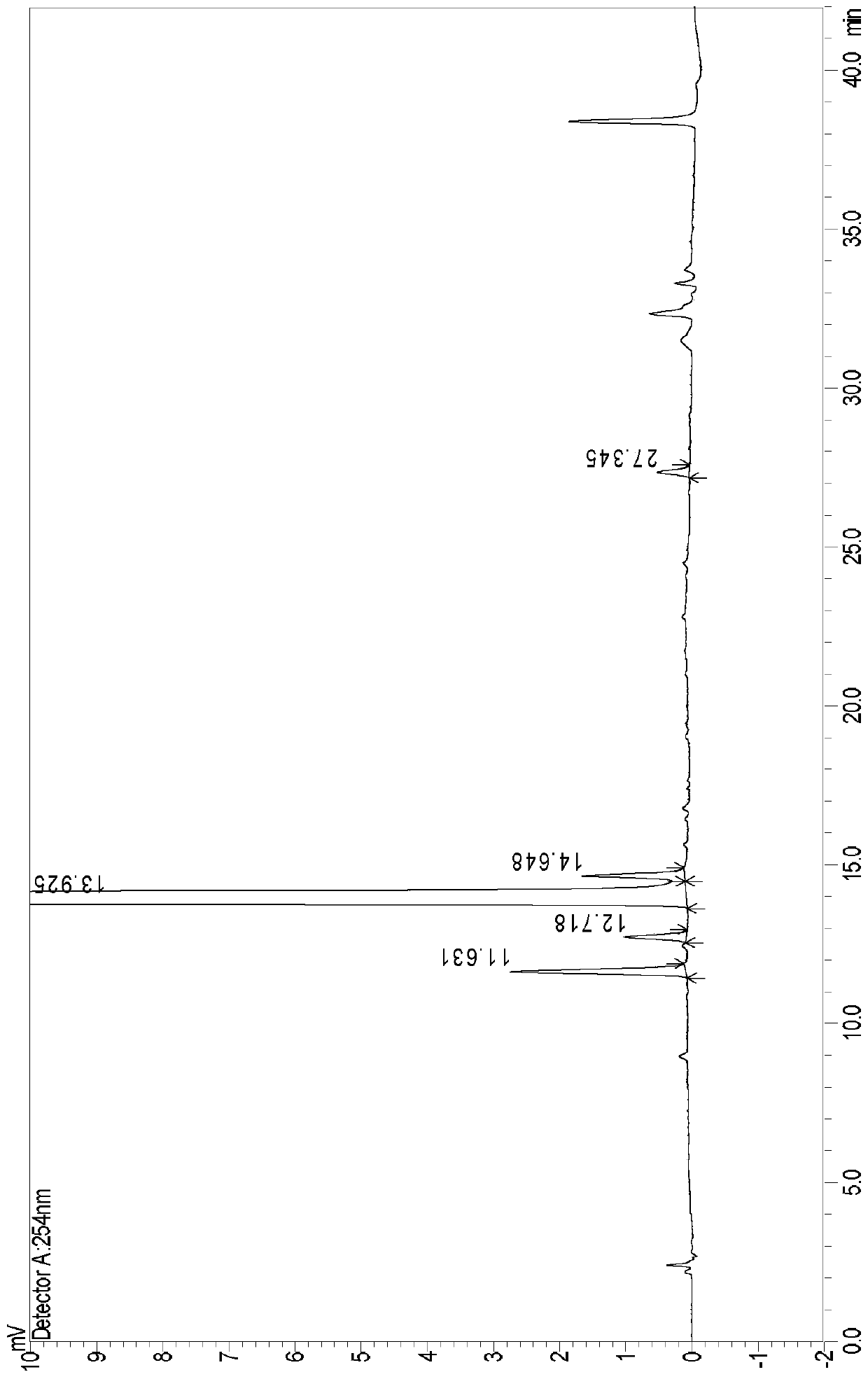
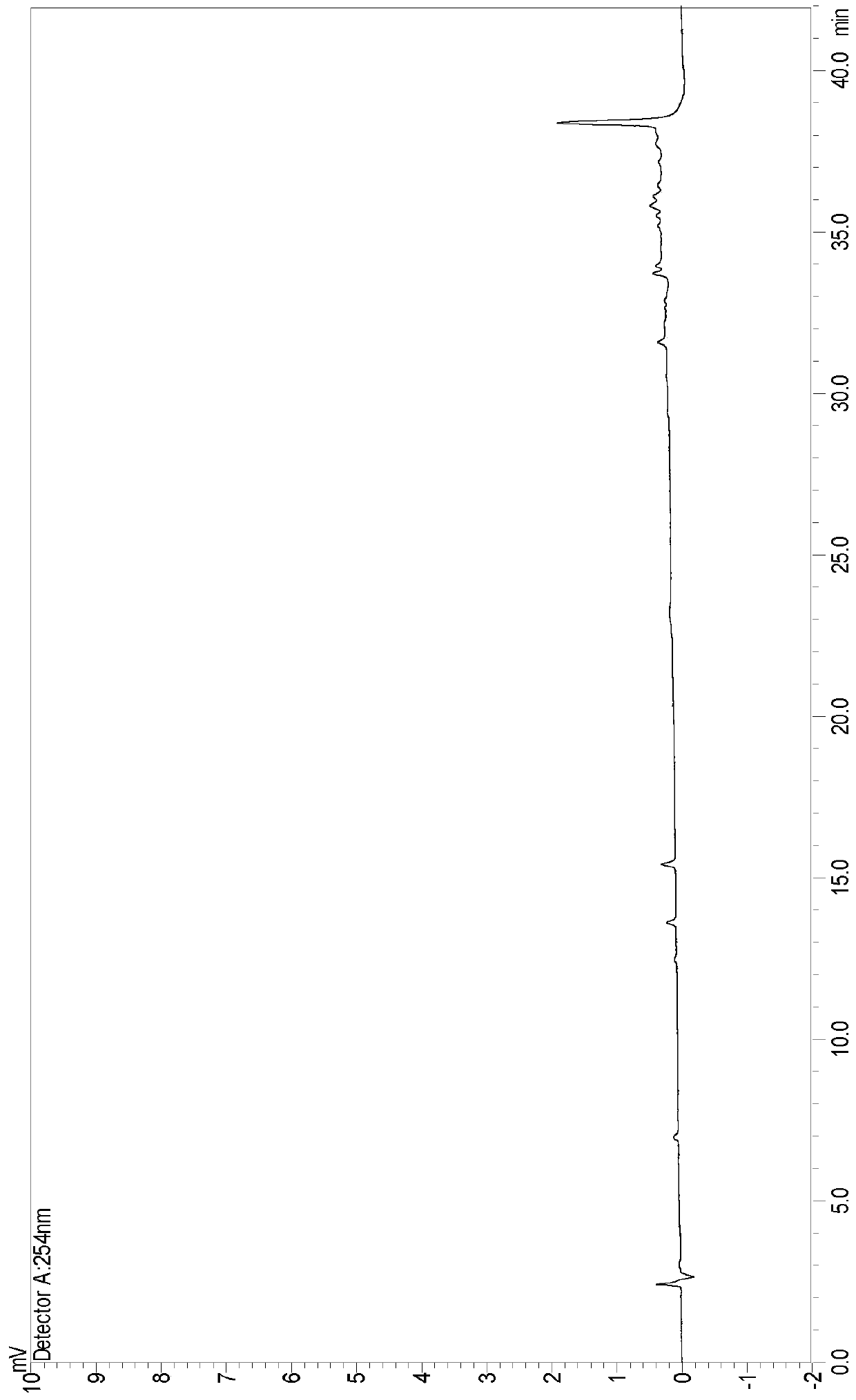
[0067] The detection of embodiment 3 desonide cream and blank matrix
[0068] Take 15g of this product, put it in a 50ml brown measuring bottle, add 15ml of 0.1% acetonitrile phosphoric acid, sonicate for 15min and shake it from time to time to completely disperse the gel, take it out and let it cool, add 5g of sodium chloride, shake well, let stand, take the supernatant The liquid was filtered, and the filtrate was used as the test solution of desonide cream. At the same time, take 15g of blank matrix, put it in a 50ml brown measuring bottle, add 15ml of 0.1% acetonitrile phosphoric acid, sonicate for 15min and shake it from time to time to completely disperse the gel, take it out and let it cool, add 5g of sodium chloride, shake well, let stand, and take the upper layer The clear liquid was filtered, and the filtrate was taken as the blank matrix test solution.
[0069] Accurately measure 10 μl each of the test solution and the blank substrate solution respectively, inject ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com