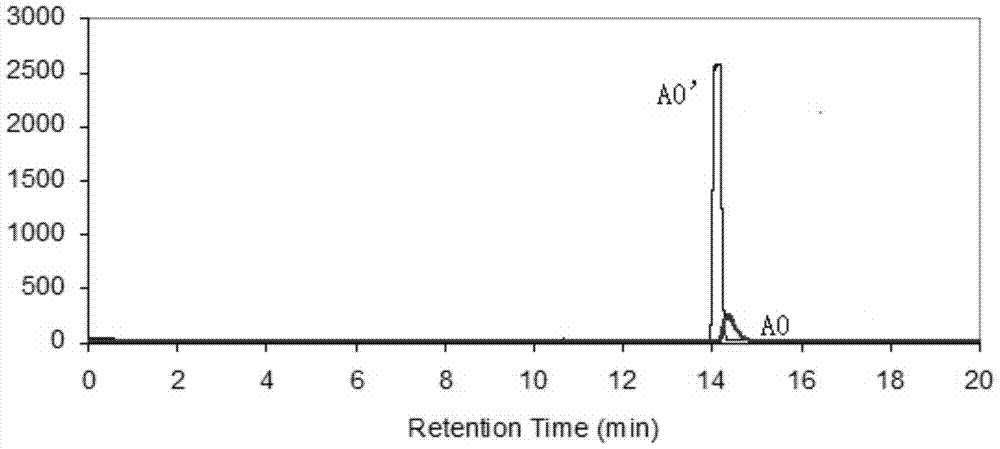
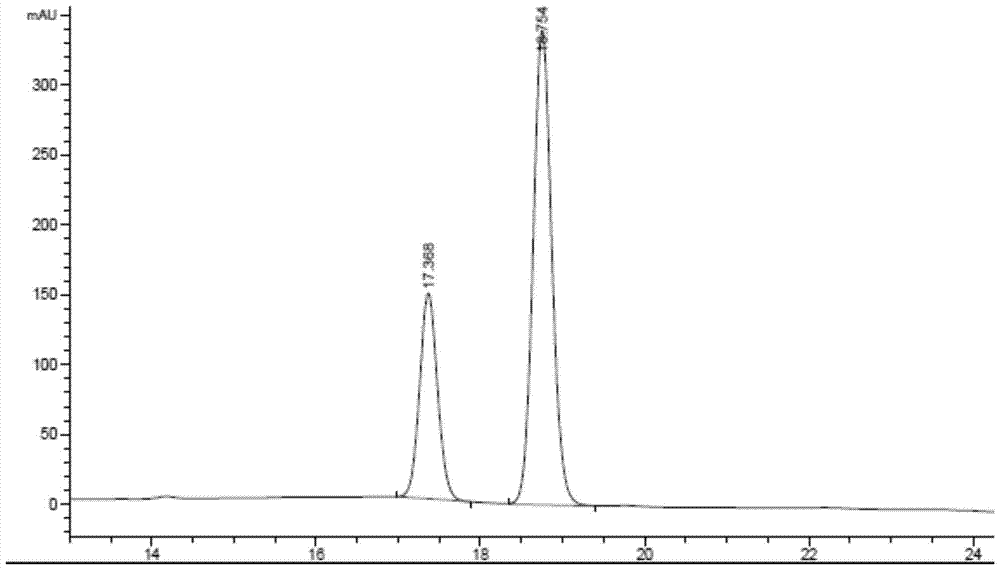
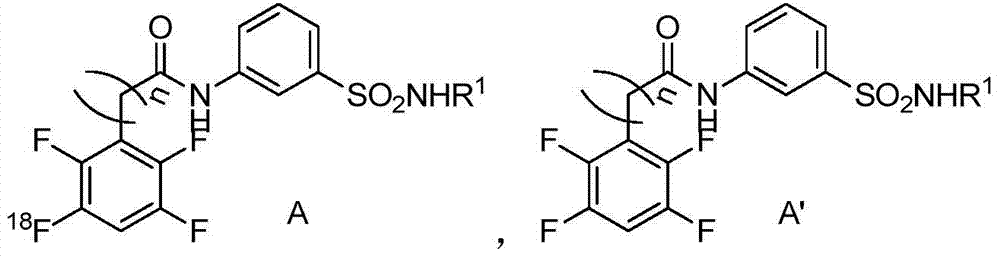
Sulfa compounds, intermediates, preparation and application targeting carbonic anhydrase ⅸ
A technology of carbonic anhydrase and compounds, applied in the preparation of sulfonamides, organic chemistry, radioactive carriers, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Synthesis of 5-nitro-2,3,6-trifluorobenzoic acid (C0)
[0077] Under ice bath, dissolve 4.0g of 2,3,6 trifluorobenzoic acid (E) in 30mL of concentrated sulfuric acid, slowly add the mixed acid solution of concentrated nitric acid (5.1mL) and concentrated sulfuric acid (5.1mL) dropwise below 0°C, add dropwise After completion, react at room temperature for 5 h. The above reaction solution was cooled in an ice bath, and 60 mL of ice water was slowly added dropwise with stirring. Extract twice with 100 mL of ethyl acetate, combine the ethyl acetate layers, wash twice with saturated sodium chloride solution, dry the organic layer over anhydrous sodium sulfate, and concentrate under reduced pressure to obtain 4.4 g of a light yellow solid. Yield 87.6%, purity >98% (HPLC).
[0078] The identification data of C0 are as follows: TOF-ESI-MS: M(C7H2F3NO4)=221.09(m / z), 244.0[M+Na]+, 276[M+Na+CH3OH]+.
[0079] 1 H-NMR (300MHz, CDCl 3 )δ8.18(m,1H,H)
Embodiment 2
[0081] Synthesis of 5-nitro-2,3,6-trifluorophenylacetic acid (C1)
[0082] 1. At room temperature, 235.7mg (1.07mmol) of CO was dissolved in 5mL of SOCl2, refluxed at 80°C for 5h, and the reaction solution was concentrated under reduced pressure to obtain light yellow liquid F, which was directly used in the next reaction.
[0083] 2. Dissolve F prepared in 1 above in 5 mL of ether, add 120 mg (2.14 mmol) of CaO, and slowly add CH2N2 (2.14 mmol) in ether solution dropwise under ice-cooling to react. After the reaction, the product was purified by silica gel column chromatography (developing solvent: n-hexane, ethyl acetate). 233.7 mg of product G was obtained with a yield of 89.1% and a purity of 99% (HPLC).
[0084] 3. 123.0mg (501.8umol) of compound G was dissolved in 5mL of dioxane solution, and 9mL ( 500mmol) of the silver oxide catalyst solution, heated at 50°C for reaction. After the reaction, the product was purified by silica gel column chromatography (developing so...
Embodiment 3
[0088] Preparation of labeled precursor compound B0
[0089] 68.9 mg (0.4 mmol) of sulfanilamide, 88.5 mg (0.4 mmol) of CO were dissolved in 6 mL of acetonitrile, 76.7 mg (0.4 mmol) of EDC.HCl and 56 uL (0.4 mmol) of triethylamine were added and the reaction was stirred at room temperature. After the reaction, the product was purified by silica gel column chromatography (developing solvent: n-hexane, ethyl acetate). The product B0125.2 mg was obtained with a yield of 83.4% and a purity of 99% (HPLC).
[0090] The identification data of B0 are as follows: TOF-ESI-MS: M(C13H8F3N3O5S)=375.28(m / z), 398.0[M+Na]+, 430.0[M+Na+CH3OH]+.
[0091] 1H-NMR (300MHz, CDCl3): δ11.36(s, 1H, NH), 8.67(m, 1H, CH), 8.23(s, 1H, CH), 7.75(m, 1H, CH), 7.59(m ,2H,CH),7.43(s,2H,NH2).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com